Application of 3D printing in early phase development of pharmaceutical solid dosage forms
Abstract
Three-dimensional printing (3DP) is an emerging technology, offering the possibility for the development of dose-customized, effective, and safe solid oral dosage forms (SODFs). Although 3DP has great potential, it does come with certain limitations, and the traditional drug manufacturing platforms remain the industry standard. The consensus appears to be that 3DP technology is expected to benefit personalized medicine the most, but that it is unlikely to replace conventional manufacturing for mass production. The 3DP method, on the other hand, could prove well-suited for producing small batches as an adaptive manufacturing technique for enabling adaptive clinical trial design for early clinical studies. The purpose of this review is to discuss recent advancements in 3DP technologies for SODFs and to focus on the applications for SODFs in the early clinical development stages, including a discussion of current regulatory challenges and quality controls.
Introduction
Three-dimensional printing (3DP) synonymous with additive manufacturing (AM), is a rapid manufacturing technology that utilises computer-aided design (CAD) in the fabrication of physical objects in a layer-by-layer (LbL) manner (Ngo et al., 2018). The advent of 3DP has the potential to cause a paradigm shift in pharmaceuticals and clinical pharmacy practice; evidence of a transition from the traditional mass production of medicines has been identified, along with a distinct move towards more tailored drug products, personalised to the patient (Vaz and Kumar, 2021). Pharmaceutical applications of 3DP have increased over the past years, offering up contemporary opportunities. This includes the manufacturing of medical devices and ‘printlets’ a term that refers to 3D printed solid oral dosage forms (SODFs) (e.g., tablets and capsules) (Jamróz et al., 2018). 3DP might provide a flexible drug-manufacturing platform, allowing for dose flexibility, drug release profiles, polypill combinations, and the efficiency of printing multiple prototypes (Jamróz et al., 2018). While some of these concepts can be developed using conventional manufacturing methods (eg.,tabletting), it is worth noting that the development process can be complex and time-consuming (Seoane-Viaño et al., 2021b) (Table 1). Advancements in 3DP within the field of pharmaceutics have already made their mark. Spritam (levetiracetam), an anti-epileptic drug, was the first 3D printed orodispersible tablet to be approved by the US Food and Drug Administration (FDA) in August 2015, produced by Aprecia Pharmaceuticals.
This 3D-printed anti-epileptic drug was revolutionary, providing an alternative way to mass manufacture Spritam tablets. Spritam is an orally disintegrating tablet (ODT) that includes a fast disintegration rate (11 sec) even with extremely high doses, which is normally challenging to obtain using traditional direct compression devices (Yang and Kim, 2023). Since setting this landmark, pharmaceutical 3DP research has displayed rapid development, such as the use of 3D printing technology to create customized orodispersible films for drug delivery, which dissolve rapidly in the mouth and offer a patient-friendly alternative for those with difficulty swallowing pills (Jamróz et al., 2017). In more recent developments, Triastek, Inc. a China-based pharma company recently received FDA clearance for its Investigational New Drug (IND) application for a 3D-printed medicine T21 (Alqahtani et al., 2023). However, despite these notable developments over the last few years, it is important to acknowledge that Spritam remains the only commercially available 3D-printed pharmaceutical printlet (Bácskay et al., 2022). Challenges persist in this emerging field. The FDA and other regulatory bodies have yet to fully approve and establish guidelines for 3D-printed tablet mass production and there are a few reasons why this is the case. Moreover, most of the printers are designed for the plastic industry and not pharmaceuticals; therefore, more research in the field in collaboration between printing developers and the pharma industry, will be needed. Additionally, it is currently more expensive than traditional tablet manufacturing methods, which can limit the scalability (Park et al., 2019).
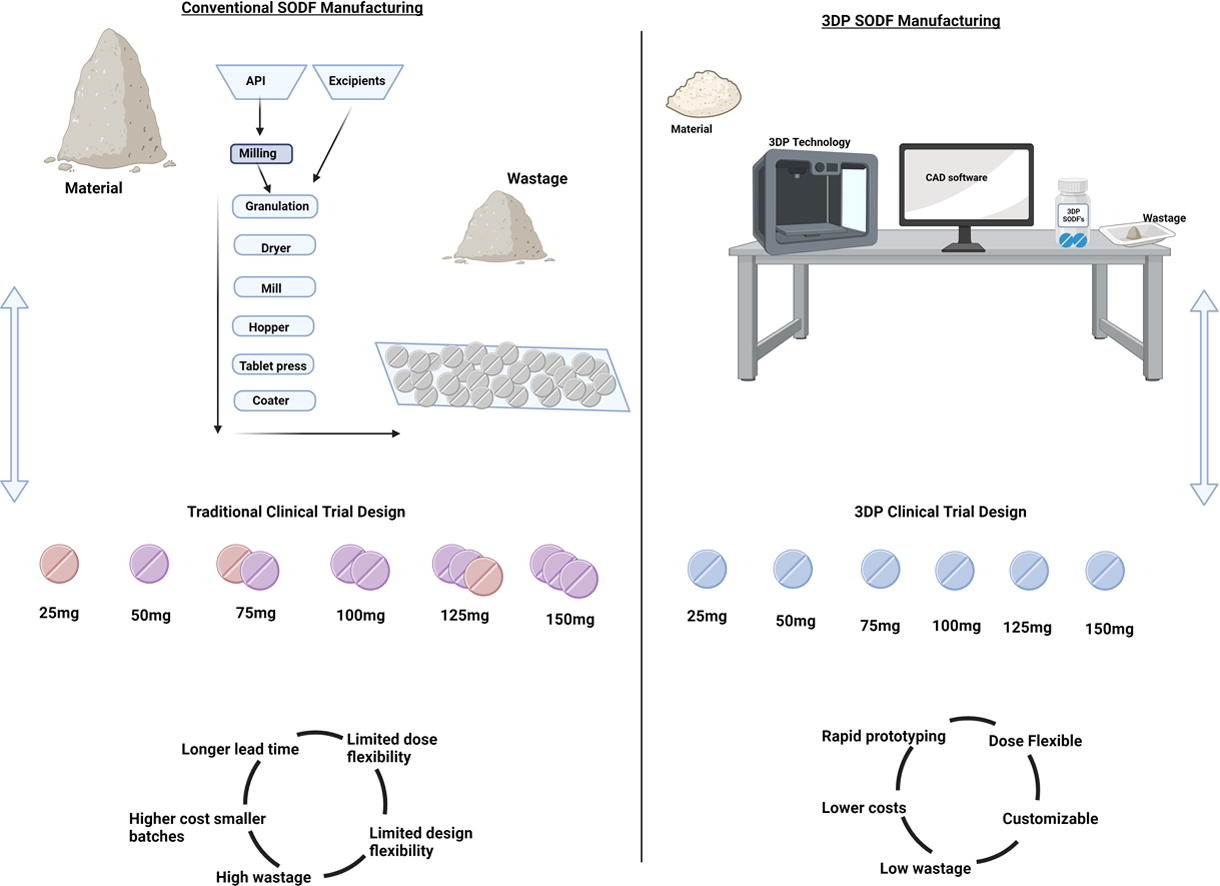
For pharmaceuticals, it is well understood that SODFs such as capsules and tablets, in addition to orally disintegrating tablets, are the most convenient and preferred route of administration (Krueger et al., 2022). It has been forecast that the oral drug delivery market will increase to $148.2 billion by 2027 from $98.3 billion in 2020 (ltd, n.d.) However, the provision of suitable tailored dosages, especially for the paediatric and geriatric populations, remains a challenge. Tablets therefore frequently require manipulation by physicians and relatives, such as having to divide tablets by hand, using knives or using a specific tablet splitter (Januskaite et al., 2020). 3DP has many advantages for producing SODFs that conventional manufacturing does not have. In particular 3DP allows parameters such as dose, shape, size, release profiles along with visual and textural aesthetics to be readily customised (Krueger et al., 2022). In today’s pharmaceutical industry, bringing a new drug to market can take more than a decade and at an estimated cost of $2.6 billion, with a low chance of a successful outcome (“Modern Drug Commercialization”, n.d.). Therefore, success within the early drug development stages is critical to the saving of both time and money. Due to its flexibility and adaptability, 3DP could be implemented to streamline, automate and accelerate the manufacturing of dosage forms in the drug development stages (Zheng et al., 2020). While many papers postulate that 3DP might replace conventional tablet manufacturing, it is a well-known fact that 3DP technology cannot compete with the mass production required. For instance, current 3D printers can only process a few hundred tablets per hour, while in comparison a high-speed tablet press are capable of manufacturing up to 240,000 tablets per hour (Elkasabgy et al., 2020).
This glaring contrast in production rates highlights the inherent limitations of 3DP technology when it comes to meeting the demands of large-scale pharmaceutical manufacturing. However, it’s important to note that 3DP needs to compete on its ability to create a wide range of customised items rather than focusing solely on production capacity. Additionally, studies are still lacking to demonstrate that similar drug release profiles can be achieved with 3D printed tablets compared to traditional tablets (i.e., ODTs). This highlights a critical gap in the current literature regarding the performance of 3D printed dosage forms in comparison to their conventionally manufactured counterparts. Where pharmaceutical companies could take advantage of the 3DP method would be in applications in which mass production is not required (Dong et al., 2022), for example, in the flexible production of small batches to support adaptive clinical trial design to facilitate clinical studies, while saving time and costs (Tracy et al., 2023). Investigating the use of 3D printing (3DP) in early pharmaceutical development is important. This becomes particularly relevant when material availability is limited, as is often the case in the early stages of drug development. During this phase, it is not only preferable but essential to screen as many concepts and strengths as possible to gain a comprehensive understanding of a candidate molecule’s behaviour. The formulation’s advantages are also dynamic, continually changing as researchers from other fields of science try to understand how the molecule functions. Due to its adaptability and flexibility, 3DP stands out as a powerful tool in this situation. It helps pharmaceutical researchers to adaptably create small batches of therapeutic dosage forms, considering the continuously shifting scientific knowledge base. This flexibility not only supports adaptive clinical trial designs but also allows pharmaceutical companies to stay agile and responsive to emerging knowledge.
By leveraging 3DP in early development, companies can efficiently explore a range of formulations, making the most of limited materials and aligning with the dynamic nature of pharmaceutical research. The strategic implementation of roadmaps emerges as a pivotal solution to overcome the challenges associated with the intellectualization and industrialization of 3D printing (3DP). Serving as a vital compass, roadmaps navigate the intricate landscape of 3DP, guiding the way toward advancements and seamless industrial integration. In the field of SODFs, this strategic approach takes on heightened significance. In addition to providing guidance for the advancement of 3DP technologies, the roadmap also directs their implementation in the accurate and effective creation of SODFs. Fundamentally, the integration of 3DP and SODFs with roadmaps presents a promising path forward, marking the beginning of a new era in pharmaceutical manufacturing that will be defined by improved intellectualization, streamlined industrial procedures, and the production of superior, patient-focused pharmaceuticals (Tian et al., 2022).This review will provide a brief overview of 3DP, with a timely perspective on the latest developments of 3DP technologies for SODFs and focus on the applications that can be used to produce SODFs for the early clinical development stages within the pharmaceutical sector. The primary objective is to critically analyse the existing literature to identify any gaps or restrictions on the use of 3DP in pharmaceutical contexts. This analysis delves into various aspects, including the practical implementation of 3DP, common misconceptions, and areas where further research may be required.
Download the full article as PDF here Application of 3D printing in early phase development of pharmaceutical solid dosage forms
or read it here
Rachel L. Milliken, Thomas Quinten, Sune K. Andersen, Dimitrios A. Lamprou, Application of 3D printing in early phase development of pharmaceutical solid dosage forms, International Journal of Pharmaceutics, 2024, 123902, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.123902.
Read more on 3D Printing here:


