Development of a New Bioequivalent Omeprazole Product
Abstract
Introduction
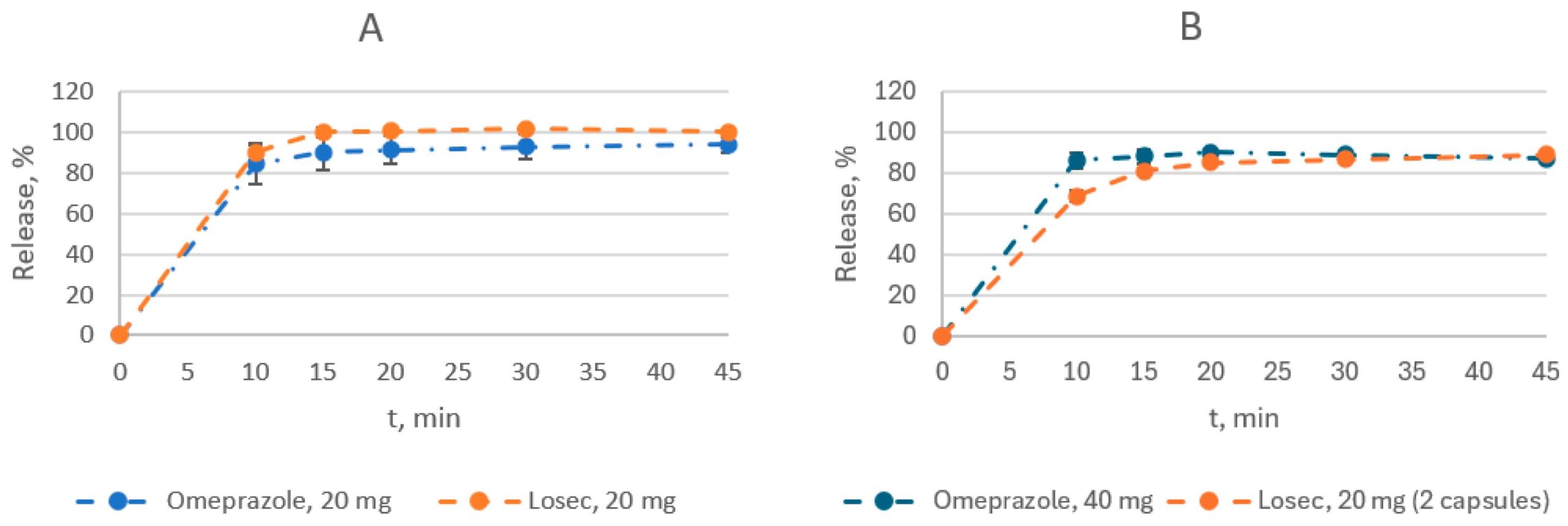
There have been several inventions aimed at producing enteric-coated pellets of PPIs. These pellets consist of an empty pellet core, a drug-loaded layer, isolating layers I and II, and an enteric-coated layer coated using a fluidized bed coater [10,11,12]. Against this background, the development of an enteric form of omeprazole in Kazakhstan has been of great interest. However, the coating of pellets or granules is a complex process that requires a high level of technology. The technical capacity to produce such products on an industrial scale is not available in all manufacturing locations. This article describes the technologically feasible development of a bioequivalent extended-release generic product containing 20 mg and 40 mg of omeprazole, focusing on an in vivo bioequivalence study.
Materials
The following materials were used in the study: omeprazole (Hetero Labs, Hyderabad, India) Active Pharmaceutical Ingredient (API), lactose monohydrate Supertab 22AN (Glentnam Life Sciences Ltd., Corsham, UK), sodium lauryl sulfate (RNDr Kulich Pharma s.r.o., Hradec Králové, Czech Republic), disodium hydrogen phosphate dodecahydrate cryst. EMPROVE® (Merck KGaA, Darmstadt, Germany), hydroxypropylmethylcellulose (Tailopur 603), hydroxypropyl cellulose Klucel EF Pharm (Ashland Industries Europe GmbH, Schaffhausen, Switzerland), pellets from microcrystalline cellulose (MCC) Cellets®700 (IPC Process center GmbH & Co.Kg, Dresden, Germany), polyethylene glycol 400 (Applichem GmbH, Darmstadt, Germany), methacrylic acid–ethyl acrylate copolymer Eudragit® L30-D55 (Evonik Nutrition and Care GmbH, Darmstadt, Germany), hard gelatin capsules size 0 (Capsugel, Bornem, Belgium), PlasAcryl® HTP20 (Emerson Resources Ink., Norristown, PA, USA), titanium dioxide (Venator Germany GmbH, Krefeld, Germany), talc (Imerys Talc Italy S.p.A, Porte, Italy). All ingredients were of pharmaceutical production grade as described in the European Pharmacopoeia, and are widely used in preparations for oral use at concentrations not exceeding the recommended limits. Losec®, enteric capsules, 20 mg, AstraZeneca AB, Sweden, was used as the reference drug.
Read also our introduction article on Talc here:


