Comparative Dissolution of Budesonide from Four Commercially Available Products for Oral Administration: Implications for Interchangeablity
ABSTRACT
Budesonide is a corticosteroid regularly used in oral formulations to treat various inflammatory diseases in the
gastrointestinal tract, such as Crohn’s disease and ulcerative colitis. Budesonide has also been formulated to be effective against immunoglobulin A (IgA)-related nephropathy. This study aimed to compare the release of budesonide from four oral formulations under discriminating dissolution conditions to ascertain if they are interchangeable. Each product had a unique dissolution profile along with differences in indications, doses, and dosing conditions. As such, there is no basis for considering the tested products to be pharmaceutically or therapeutically interchangeable.
INTRODUCTION
Budesonide is a corticosteroid regularly used in oral formulations to treat various inflammatory diseases in the gastrointestinal (GI) tract, such as Crohn’s disease and ulcerative colitis. Because these inflammatory conditions affect different parts of the GI tract, different formulations have been introduced over the years to deliver budesonide selectively to inflamed tissue.
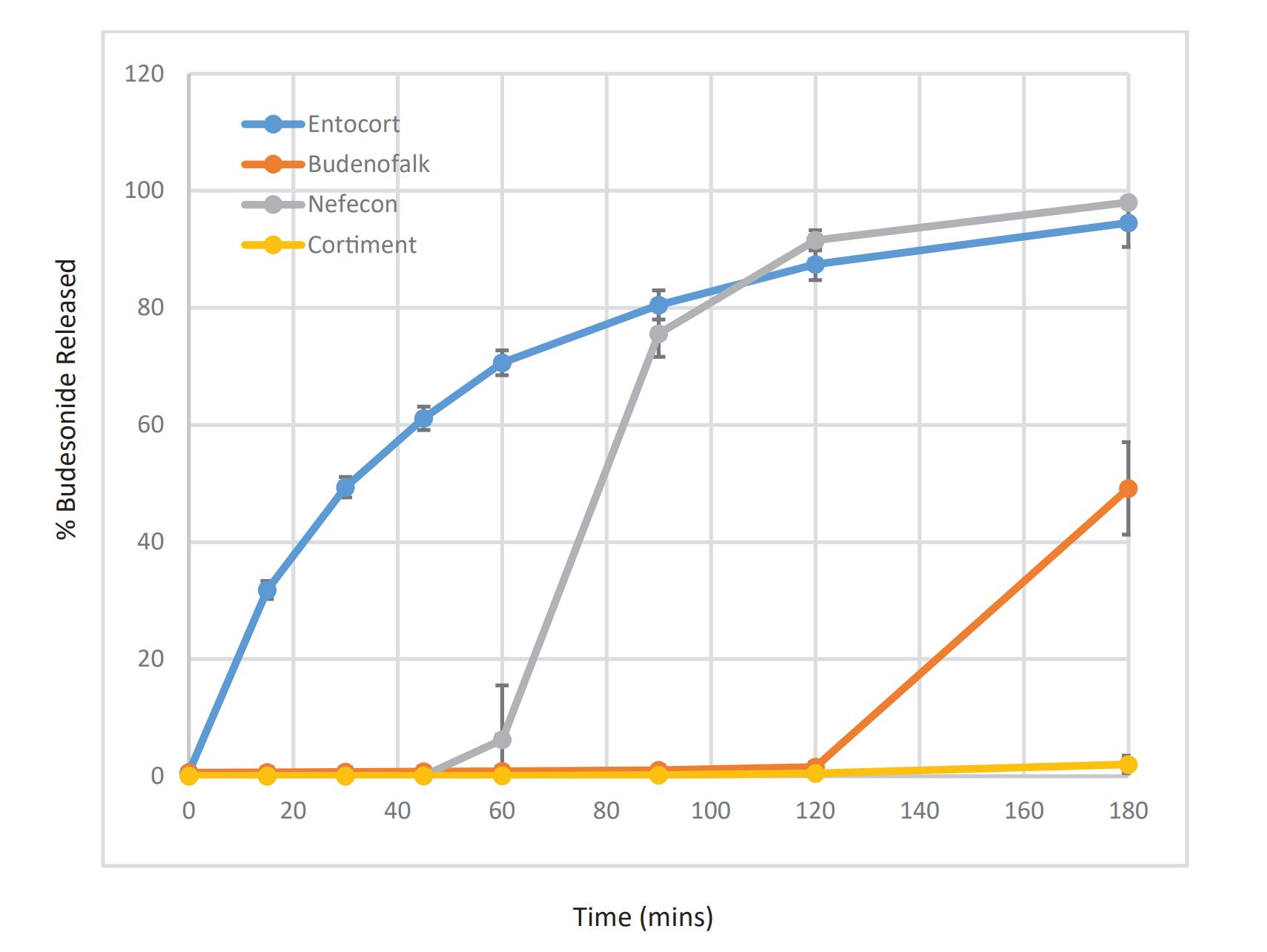
Entocort EC is indicated for treatment of active mild to moderate Crohn’s disease at a daily dose of 9 mg (three 3-mg capsules once daily) or 6 mg (two 3-mg capsules in the morning) to maintain patients in remission (1). The equivalent European product, Entocort has similar indications, with the addition of microscopic colitis (2). Although Entocort is approved for treatment of the disease in both the ileum and ascending colon, the capsules consist of beads with an enteric coating (Eudragit L55, Evonik GmbH, Germany) designed to dissolve at pH 5.5 and above, so budesonide release likely starts proximally in the small intestine. Once the enteric coating on the beads is dissolved, an ethylcellulose component provides sustained release of budesonide.
Budenofalk (currently marketed in Europe but not available in the USA) is indicated for induction of remission in patients with mild to moderate active Crohn’s disease affecting the ileum and/or the ascending colon at a daily dose of 9 mg (either three 3-mg capsules in the morning or one capsule in the morning, one at midday, and one in the evening). Budenofalk has also been approved for the treatment of microscopic colitis and autoimmune hepatitis (3). Like Entocort EC, the oral formulation consists of enterically coated beads housed in a capsule, but the coating is a mixture of Eudragit L and Eudragit S. The combination of Eudragit L and S in the coating formulation has a higher nominal pH of release (pH 6.4) than that of Eudragit L alone, so budesonide release from Budenofalk likely begins more distally in the small intestine than from Entocort EC (4). Once the enteric coating components on the bead dissolve, prolonged release of the active component is provided by Eudragit RS.
Table 1. Pharmaceutical Characteristics of Delayed-Release Budesonide Oral Formulations
| Parameter | Nefecon | Budenofalk | Entocort | Cortiment |
|---|---|---|---|---|
| Enteric coating material and component | Eudragit L and S on capsule shell | Eudragit L and S on beads | Eudragit L55 on beads | Eudragit L55 and S on tablet |
| Nominal pH of enteric coating | Proprietary information a | pH 6.4 (RMS Assessment Report) | pH 5.5 (FDA) | pH 7 (FDA) |
| Capsule material | HPMC | Gelatin | Gelatin | N/A |
| Sustained-release component | Ethylcellulose-based coating on beads | Eudragit RS | Ethylcellulose | MMX (stearic acid/HPC matrix) |
aNominal pH is between that of Entocort and Budenofalk (written communication, Calliditas Therapeutics).
RMS: Regulatory Management System of the Medicines and Healthcare Products Regulatory Agency (MHRA), UK; HPMC: hydroxypropyl methylcellulose; MMX: multimatrix formulation; HPC: hydroxyproplycellulose.
Cortiment 9-mg prolonged-release tablets are approved for induction of remission in patients with mild to moderate active ulcerative colitis (UC) in cases where 5-aminosalicylic acid (ASA) treatment is not sufficient, as well as to induce remission in adult patients with active microscopic colitis. Tablets are taken with or without food in the morning, taking care not to break, crush, or chew the tablet, because the film coating is intended to ensure a prolonged release (5). However, because the coating is a mixture of Eudragit L and S with a release pH of 7, it serves more as a delaying rather than a prolonging component. Prolonged (extended) release of budesonide is ensured by embedding the drug in a multimatrix (MMX) formulation. The applications of MMX formulations have been described (6).
Nefecon (TARPEYO in US; Kinpeygo in UK) 4-mg delayed release capsules are another oral product containing budesonide. Unlike the other commercially available formulations, Nefecon is indicated to reduce urine protein levels in adults with primary immunoglobulin A (IgA) nephropathy who are at risk of rapid disease progression (7). Nefecon is formulated to target the release of budesonide to gut-associated lymphoid tissue, in particular the Peyer’s patches (8). Four capsules are taken in the morning 1 hour before food intake. Instead of enterically coated beads, the capsule itself is coated. The beads containing budesonide are housed in the capsule, and release from the beads is regulated by an ethylcellulose-based coating.
The aim of this study was to compare release of budesonide from these four products under discriminating dissolution conditions to ascertain if they are interchangeable in terms of delivering budesonide to the same location within the GI tract.
Download the full study as PDF here Comparative Dissolution of Budesonide from Four Commercially Available Products for Oral Administration
Source: Jennifer Dressman Fraunhofer Institute of Translational Medicine and Pharmacology, Frankfurt, Germany, Comparative Dissolution of Budesonide from Four Commercially Available Products for Oral Administration: Implications for Interchangeablity
Read more on “Orally Disintegrating Tablets (ODTs)” here:


