Continuous direct compression: Development of an empirical predictive model and challenges regarding PAT implementation
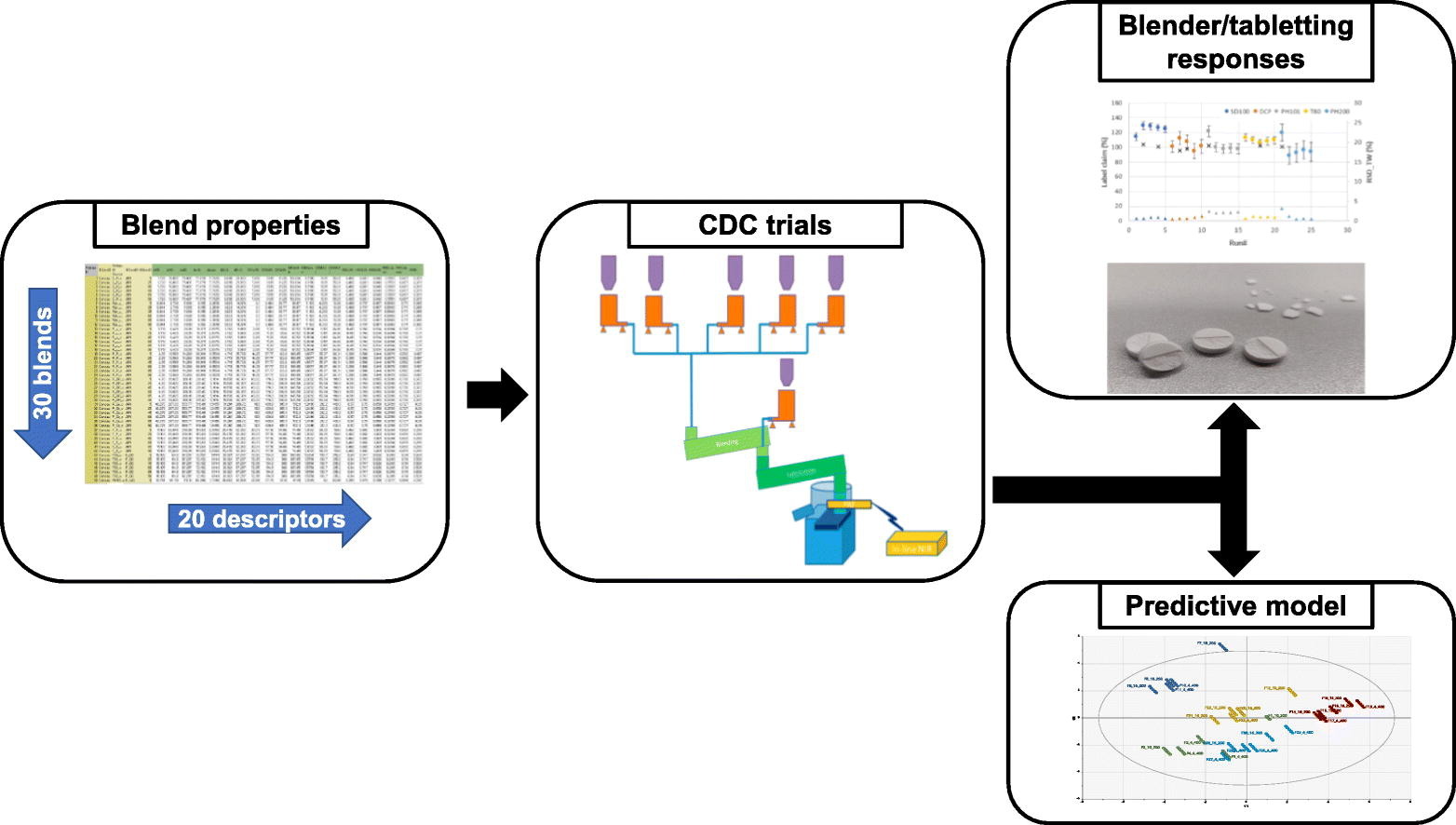
In this study, an empirical predictive model was developed based on the quantitative relationships between blend properties, critical quality attributes (CQA) and critical process parameters (CPP) related to blending and tableting. The blend uniformity and API concentration in the tablets were used to elucidate challenges related to the processability as well as the implementation of PAT tools. Thirty divergent ternary blends were evaluated on a continuous direct compression line (ConsiGma™ CDC-50). The trials showed a significant impact of the impeller configuration and impeller speed on the blending performance, whereas a limited impact of blend properties was observed. In contrast, blend properties played a significant role during compression, where changes in blend composition significantly altered the tablet quality. The observed correlations allowed to develop an empirical predictive model for the selection of process configurations based on the blend properties, reducing the number of trial runs needed to optimize a process and thus reducing development time and costs of new drug products. Furthermore, the trials elucidated several challenges related to blend properties that had a significant impact on PAT implementation and performance of the CDC-platform, highlighting the importance of further process development and optimization in order to solve the remaining challenges.
Download the full research paper as PDF: Continuous direct compression – Development of an empirical predictive model and challenges regarding PAT implementation
or read the article here
B. Bekaert, B. Van Snick, K. Pandelaere, J. Dhondt, G. Di Pretoro, T. De Beer, C. Vervaet, V. Vanhoorne,
Continuous direct compression: Development of an empirical predictive model and challenges regarding PAT implementation,
International Journal of Pharmaceutics: X, 2021, 100110, ISSN 2590-1567,
https://doi.org/10.1016/j.ijpx.2021.100110.
Materials:
| Paracetamol powder | Mallinckrodt | P_P |
| Paracetamol dense powder | Mallinckrodt | P_DP |
| Paracetamol micronized | Mallinckrodt | P_μ |
| Caffeine anhydrous powder | BASF | C_P |
| Metoprolol tartrate micronized | Utag | MPT_μ |
| Theophylline anhydrous powder | Siegfried | T_P |
| Spray dried API | Janssen | API_sd |
| Pearlitol 100 SD | Roquette | SD100 |
| Emcompress AN | JRS | DCP |
| Avicel PH-101 | FMC | PH101 |
| Avicel PH-200 | FMC | PH200 |
| Tablettose 80 | Meggle | T80 |
| Ligamed MF-2-V | Peter Greven | MgSt |

