Formulation and evaluation of dabigatran loaded microballoons for prolonged drug release
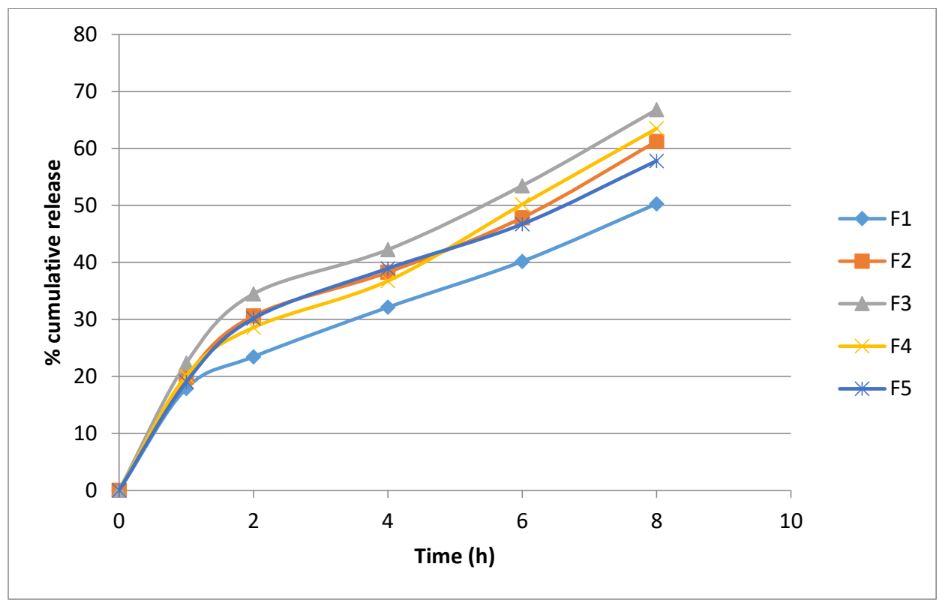
The objective of the present investigation was to formulate microballoons loaded with dabigatran in the polymeric core for achieving sustained release and hence improved bioavailability. Microballoons loaded with dabigatran in the polymer shell were prepared by simple solvent evaporation method using either ethyl cellulose alone or a blend of ethyl cellulose with HPMC/PVP-K30/Eudragit S100/Methylcellulose in a fixed ratio. The percent yield of the microballoons ranged from 38.7 to 62.4% with highest yield obtained in F3. The particle size was measured using optical microscopy and the particles were observed to be spherical in shape. The particle size ranged from 28.50 ± 16.378 µm (F3) to 48.14 ± 16.748 µm (F1). The angle of repose ranged from 26.37° to 28.09° and the Carr’s index and Hausner’s ratio were in between 6.38 to 19.95 and 1.07 to 1.25 respectively. It was found that all the formulations exhibited buoyancy in the range of 61.42 to 71.71% over a period of 8h. This suggests that the formulations were able to float for sufficient time and would be able to control the release of dabigatran for longer duration. The in vitro drug release study depicted that the highest amount of drug was released from F3 (66.79%) while the lowest was released from F1 (50.27%) at the end of 8 hours of study.
1. Introduction
Traditional oral dosage forms such as tablets and capsules provide a specific drug concentration in systemic circulation but they are not able to release the drug at a constant rate for a longer duration of time.1 Gastro retentive drug delivery is based on design and development of systems that might be able to retain the dosage form in the stomach for longer time periods. These systems are particularly beneficial in improving the bioavailability of drugs that possess low solubility in the high pH environment (like intestine).Microballoons are the gastro retentive drug delivery system and it is based on the non-effervescent approach usually possessing spherical shape and lack a core with a particle size of less than 200 micrometer.Dabigatran etexilate is an anticoagulant used for the prevention of venous thromboembolic events or stroke in patients with recent elective hip or knee replacement surgery and atrial fibrillation.5,6 Dabigatran is a univalent reversible direct thrombin inhibitor (DTI) that competitively inhibits thrombin. Additionally dabigatran has also been shown to inhibit platelet aggregation, another step in the coagulation pathway. It has an oral bioavailability of 3 to 7% and a half-life of 12-15 h. The objective of this work was the development and investigation of floating microspheres (microballoons) of dabigatran to modulate its pharmacokinetic profile and increase its half-life. Treatment of disease requires maintenance of uniform concentration of drug in blood for a long period of time. Floating microspheres were envisaged as the most promising drug delivery system owing to their slow dissolution in gastric fluid thereby rendering the capability to prolong the release of drug at the site of absorption.
| Ingredients | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|
| Dabigatran (mg) | 100 | 100 | 100 | 100 | 100 |
| EC (mg) | 2000 | 1500 | 1500 | 1500 | 1500 |
| HPMC (mg) | 0 | 500 | 0 | 0 | 0 |
| PVP-K30 (mg) | 0 | 0 | 500 | 0 | 0 |
| Eudragit S100 (mg) | 0 | 0 | 0 | 500 | 0 |
| Methyl cellulose (mg) | 0 | 0 | 0 | 0 | 500 |
Download the full study as PDF here: Formulation and evaluation of dabigatran loaded microballoons for prolonged drug release
Aryan Jain, Amit Jain, Formulation and evaluation of dabigatran loaded microballoons for prolonged drug release, Journal of Propulsion Technology, Vol. 44 No. 3 (2023), ISSN: 1001-4055

