Implementing the Design of Experiments (DoE) Concept into the Development Phase of Orodispersible Minitablets (ODMTs) Containing Melatonin
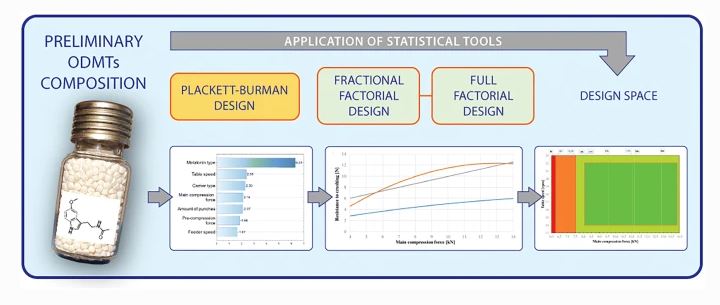
Development of orodispersible minitablets (ODMTs) requires consideration of aspects related to small dimensions, while ensuring short disintegration time with sufficient mechanical stability. In order to meet these and other critical quality attributes (CQAs), quality by design is encouraged. According to this approach, formulation and compression process factors were systematically studied using design of experiments (Plackett-Burman for screening purposes, full and fractional factorial design for in-depth characterization) to understand their influence on CQAs of orodispersible minitablets containing melatonin.
Mathematical models describing the relationships between processing variables and attributes such as resistance to crushing and disintegration time were successfully developed, characterized by high coefficients of determination (R2adj = 0.90–0.97) and prediction errors in the range (+2.4 to −10.8%). In conclusion, based on these models, the design space was created for melatonin ODMTs, ensuring the product’s quality and process robustness. Moreover, the study demonstrated the suitability of texture analysis as an alternative to compendial measurement methods of resistance to crushing and disintegration time.
continue reading here
About this article: Hejduk, A., Teżyk, M., Jakubowska, E. et al. Implementing the Design of Experiments (DoE) Concept into the Development Phase of Orodispersible Minitablets (ODMTs) Containing Melatonin. AAPS PharmSciTech 23, 60 (2022). https://doi.org/10.1208/s12249-021-02185-6
Materials
Minitablet composition was based on two types of co-processed excipients (CPE): GalenIQ™ 721 (further in text abbreviated to GIQ) (Beneo GmbH, Germany) and Granfiller-D™ 215 (further in text abbreviated to GFD) (Daicel Corporation, Japan). Crospovidone (BASF) was selected as the disintegrant. Aspartam (Hyet Sweet S.A.S., France) was used as the sweetener agent and orange flavor type Q-128155 (Givaudan, Switzerland) was applied. Vegetable magnesium stearate (FACI, Italy) was used as a lubricant. Micronized and non-micronized (coarse grade) melatonin (Flamma S.p.A., Italy) was used as the active pharmaceutical ingredient (API). All samples were kindly provided by the manufacturers.

