Electromechanical convective drug delivery devices for overcoming diffusion barriers
Abstract
Drug delivery systems which rely on diffusion for mass transport, such as hydrogels and nanoparticles, have enhanced drug targeting and extended delivery profiles to improve health outcomes for patients suffering from diseases including cancer and diabetes. However, diffusion-dependent systems often fail to provide >0.01–1% drug bioavailability when transporting macromolecules across poorly permeable physiological tissues such as the skin, solid tumors, the blood-brain barrier, and the gastrointestinal walls. Convection-enabling robotic ingestibles, wearables, and implantables physically interact with tissue walls to improve bioavailability in these settings by multiple orders of magnitude through convective mass transfer, the process of moving drug molecules via bulk fluid flow. In this review, we compare diffusive and convective drug delivery systems, highlight engineering techniques that enhance the efficacy of convective devices, and provide examples of synergies between the two methods of drug transport.
Introduction
Diffusion-based drug delivery systems passively elute therapeutics from a high concentration formulation, such as a hydrogel, into a lower concentration environment. These biomaterials provide patients with therapies that enable: tunable sustained release profiles that reduce dosing frequencies; cell targeting capabilities that reduce off-target side effects; and high biocompatibilities that mitigate the foreign body response [[1], [2], [3]]. However, controlled release and nanomedicine formulations suffer significant hurdles during clinical translation. The disconnect between the vast number of academic papers and few clinical approvals by the US Food and Drug Administration (FDA) for nanoparticles and hydrogels has generated questions about the utility of these technologies [4,5].
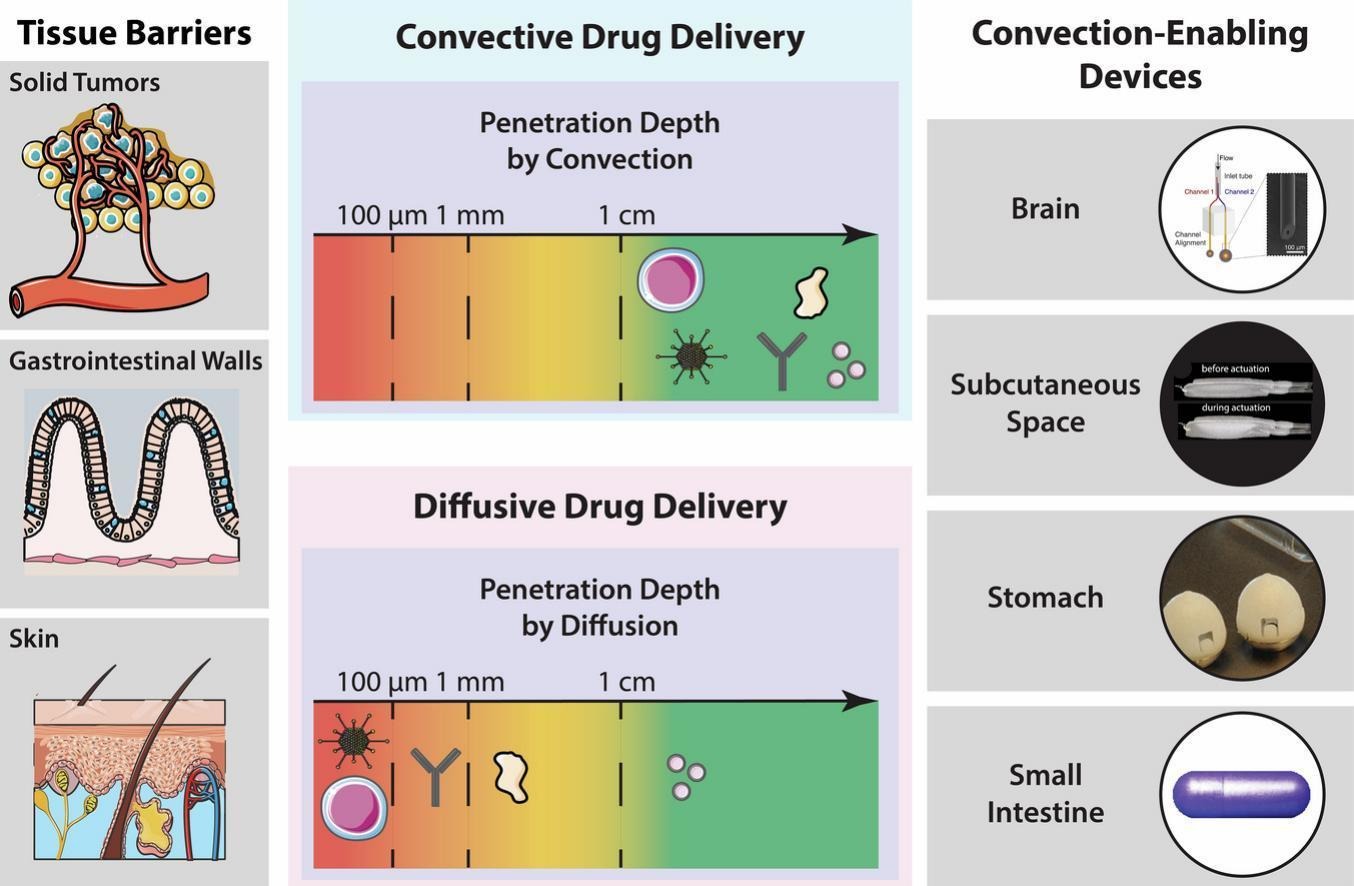
When biomacromolecular drugs need to diffuse across a poorly permeable physiological barrier, their bioavailabilities fall to 1% or less [[6], [7], [8], [9]]. For example, around 0.7% of nanoparticles reach tumors after intravenous injections [7], while the bioavailabilities of orally, intragastrically, topically, and intranasally administered peptides are approximately 1% [8], <0.1% [10], <0.1% [11], and 1–3% [6,9], respectively. Strategies to facilitate transport across physiological diffusion barriers, such as loosening tight junctions [12,13], exploiting receptor mediated transcellular transport [[14], [15], [16], [17]], ionic liquids [[18], [19], [20]], protease inhibitors [21], and permeation enhancers [22,23] have been developed, but modest diffusion coefficient improvements still keep bioavailabilities in the low single digits. Especially for drugs with injection doses over 1 mg/week, the monetary and volume constraints associated with loading 100 times as much drug hold these alternative delivery technologies back from clinical translation [24,25]. Additional complications such as initial burst releases, lack of control over spatiotemporal concentration profiles, and inability to make modifications following administration remain key challenges in the field.
To overcome the limitations of diffusion-based formulations, convection-based delivery systems transfer drugs via bulk fluid flow. This allows macromolecules to travel further into tissue, in part by physically disrupting diffusion barriers and also by utilizing pressure gradients to push formulations into narrow crevasses. In 1994, Oldfield’s group developed a novel method for drug delivery in the brain called convection-enhanced delivery (CED) [26]. By inserting catheters directly into the brain, they delivered an infusion of low and high molecular weight compounds, sucrose (359 Da) and transferrin (80 kDa), to a large volume of the brain. Immediately after two-hour infusions, sucrose and transferrin spread approximately 2 cm and 1.5 cm from their initial locations, respectively; if these molecules were distributed solely by diffusion, the estimated average depths of tissue penetration are only 0.39 cm and 0.07 cm, respectively.
Convection enhances the depth of tissue penetration by multiple orders of magnitude, with the effect being magnified for larger molecules. In addition to enhanced distribution profiles and bioavailabilities, convection-based systems can also actively regulate drug release timings, granting greater control over the drug’s spatiotemporal concentration profile [26,27]. However, developing convection-based methods requires engineering complex, invasive, and expensive electromechanical devices that localize to the targeted tissue area and insert drug into the appropriate tissue layer. For this reason, convective devices are only recently reaching the clinic.
In this Review, we describe the challenges behind engineering convection-enabling drug delivery devices, quantify the advantages these systems have over diffusion-based delivery, and highlight tissues that benefit from convection-based methods. First, we analyze mass transport models estimating tissue penetration depths of macromolecules by diffusion and convection. We describe the length scales of various physiological barriers in the body and the timescales required for macromolecules to diffuse across those barriers. Based on the models and barriers, we then discuss the clinical successes and failures of diffusion-based methods.
Next, we introduce convection-enabling devices that enhance drug delivery across barriers to enable unique targeting and release profiles. We then compare these systems to devices that physically disrupt tissue barriers but still rely on diffusion for mass transport, and we show that convective systems provide superior drug distribution over these other technologies. Finally, we highlight engineering techniques to obtain optimal drug distribution patterns by convective drug delivery. Through this analysis, we provide insight into how convective systems could augment the field of drug delivery to achieve more efficient and controlled release profiles to areas of the body where diffusion-based transport alone does not yield sufficient therapeutic efficacy.
Read the full article on Electromechanical convective drug delivery devices
Jihoon Park, Ramy Ghanim, Adwik Rahematpura, Caroline Gerage, Alex Abramson, Electromechanical convective drug delivery devices for overcoming diffusion barriers, Journal of Controlled Release, Volume 366, 2024, Pages 650-667, ISSN 0168-3659, https://doi.org/10.1016/j.jconrel.2024.01.008.

