Self-microemulsifying system of an ethanolic extract of Heliopsis longipes root for enhanced solubility and release of affinin
Abstract
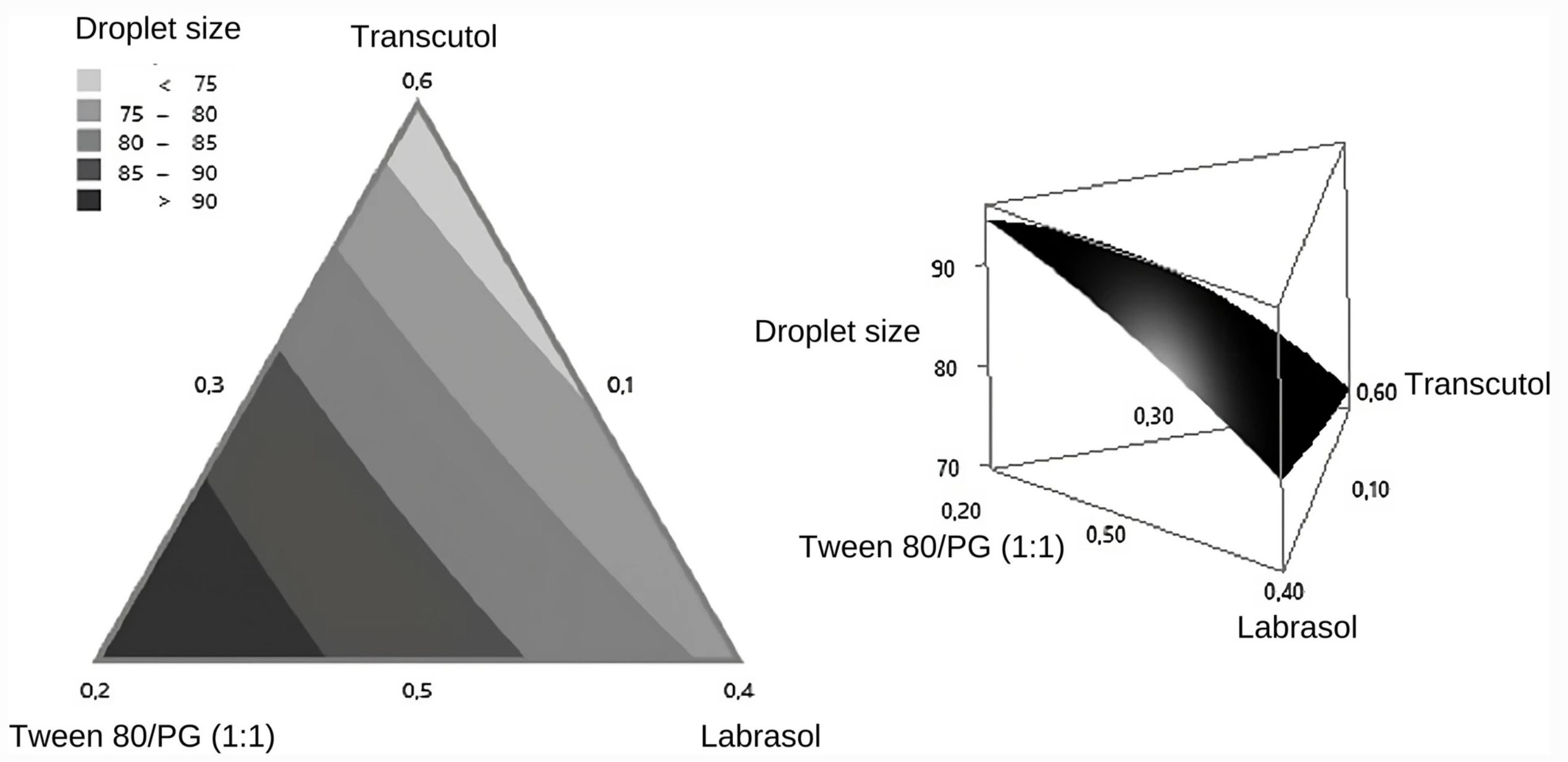
Self-microemulsifying or self-nanoemulsifying drug delivery systems (SMEDDS/SNEDDS) are well known to improve the dissolution and increase the oral bioavailability of hydrophobic drugs, including herbal extracts. Organic extracts of Heliopsis longipes root and affinin, its main component, induce a vasodilator effect; however, they are poorly water soluble and therefore are difficult to administer and dose by the oral route. This research aimed to develop, through pseudo-ternary phase diagrams, a self-microemulsifying system prepared from an ethanolic extract of H. longipes root (HL-SMDS). In addition, the optimized lipid-based formulation was characterized and its in vitro gastrointestinal simulated dissolution was determined. The formulation composed of Transcutol, 55% (solubilizer); Tween80/PG, 10% (surfactant/co-solvent); Labrasol, 35% (surfactant); and the herbal extract was selected as optimal and identified as a SMEDDS, since when coming into contact with water, it forms a micro-emulsion with droplet sizes less than 100 nm. The stability tests showed that HL-SMDS remained stable over time under extreme conditions. Furthermore, the amount of affinin released from HL-SMDS at pH 1 and 6.8 was higher than that of the ethanolic extract from H. longipes root. These results indicate that HL-SMDS is a novel alternative to improve the aqueous solubility and therefore the oral bioavailability of the ethanolic extract of H. longipes root.
Introduction
Medicinal plants have been considered over the years as the origin or starting point of the development of medicines, since they have contributed to the discovery of new bioactive compounds and the obtaining of herbal medicines. In addition, plants used in traditional medical systems are more accessible to the population, specifically those with fewer resources and poor medical services (Acosta-Recalde et al. 2018; World Health Organization (WHO) 2019). However, many of the compounds or extracts obtained from medicinal plants are poorly water-soluble, which hinders their subsequent formulation in pharmaceutical forms that would allow their administration, dosage, and bioavailability (Rohini et al. 2021; Chairuk et al. 2020).
The oral bioavailability of a drug depends on its solubility and dissolution rate in the gastrointestinal (GI) fluid. Therefore, these parameters are important factors to consider when developing drug delivery systems. In order to enhance the solubility and dissolution rate of poorly water-soluble drugs (BCS II and IV class), new formulation strategies have been proposed to improve the bioavailability of these molecules, including micronization or nanosizing, salt formation, complexation with cyclodextrins, co-solvent and surfactant-based solubilization, amorphous solid dispersions, and lipid formulations (micro- and nano-emulsions, and other lipid-based nanostructures) (Porat and Dahan 2018; Alshamrani et al. 2022; Pouton 2006).
Self-emulsifying drug delivery systems (SEDDS) are lipid-based formulations (LBFs) that emulsify (o/w) in aqueous media and generate nano-droplets, when subjected to a gentle agitation (Pouton 1997; Anton and Vandamme 2011), simulating what happens with the peristaltic movements of the gastrointestinal tract (Pouton 1997; Shah and Agrawal 2020; Oliveira and Bruschi 2022). Ideally, SEDDS are composed of isotropic mixtures of natural or synthetic oils with lipophilic surfactants (HLB: 8–12) and hydrophobic co-solvents/co-surfactants (Pouton 2006; Pouton and Porter 2008). On the other hand, the use of hydrophilic surfactants (HLB > 12), hydrophilic co-solvents, and co-surfactants would allow obtaining self-microemulsifying drug delivery systems (SMEDDS) and self-nanoemulsifying drug delivery systems (SNEDDS). The advantages of SEDDS, SMEDDS, and SNEDDS have increased their use in the last decades as an alternative to solubilize and improve the dissolution rate and bioavailability of hydrophobic drugs (Pavoni et al. 2020; Tan et al. 2021).
The composition and properties of SEDDS, SMEDDS, and SNEDDS are summarized in the Lipid Formulation Classification System (LFCS), which categorizes lipid formulations into four groups (types I, II, IIIA, IIIB, and IV), classified according to their formulation components, hydrophobicity, dispersibility, and digestability (Pouton and Porter 2008; Niederquell and Kuentz 2013; Williams et al. 2014).
Currently, some pharmaceutical journals describe SEDDS as nano-emulsions or micro-emulsions without any distinction, which generates confusion among the scientific community, despite the fact that both systems have different physical and physicochemical properties (Pavoni et al. 2020; McClements 2012). Micro-emulsions (MEs) are isotropic dispersions that are spontaneously formed when oils, surfactants, and optionally one or more co-solvents come into contact with water. They exist in a thermodynamic equilibrium that depends on the composition, temperature, and atmospheric pressure (Anton and Vandamme 2011; Pavoni et al. 2020; McClements 2012).
As previously mentioned, one of the main challenges in the formulation of new drugs is their low solubility in water. In fact, it has been estimated that around 80% of all new drug candidates are poorly soluble in water (Acharya et al. 2011; Quirino-Barreda et al. 2017; Shanley 2018), which is the case of the organic extracts obtained from Heliopsis longipes root and affinin. This species, endemic to Mexico, is employed in Mexican Traditional Medicine for the treatment of some illnesses, including toothache, gingival disease, and muscle pain (Déciga-Campos et al. 2012; Mata et al. 2019; Cariño-Cortés et al. 2010; Cilia-López et al. 2010). The major components found in H. longipes root are alkamides, mainly (2E, 6Z, 8E) -N-isobutyl-2,6,8-decatrienamide also known as affinin or spilanthol (Willig et al. 2019). Both the extracts prepared from H. longipes root, as well as affinin in its pure state, have shown different biological and pharmacological activities (Althaus et al. 2017; Barbosa et al. 2016; Rosa-Lugo et al. 2017). In addition, our research group demonstrated that affinin, and the dichloromethane and ethanolic extracts from H. longipes root induce a concentration-dependent vasodilator effect (Castro-Ruiz et al. 2017). Therefore, we hypothesized that this plant’s roots could be used to develop a useful herbal medicine to treat high blood pressure.
Considering that the organic extracts obtained from H. longipes root are lipophilic, it was necessary to find suitable formulations that would allow their administration in animal or human models and for developing herbal medicines. Thus, the present work aimed to develop, through pseudo-ternary phase diagrams, a self-microemulsifying system prepared from an ethanolic extract of H. longipes root. The optimized lipid-based formulation was characterized, and its in vitro gastrointestinal simulated dissolution was assessed.
Download the full article as PDF here Self-microemulsifying system of an ethanolic extract of Heliopsis longipes root for enhanced solubility and release of affinin
or read it here
Materials
Reagents and solvents (absolute ethanol, acetonitrile, acetic acid, hydrochloric acid, monobasic potassium phosphate, sodium hydroxide, carboxymethylcellulose) used in the present investigation (A.R. and HPLC grade) were obtained from JT Baker® (Phillipsburg, NJ, USA). Double-distilled water was used throughout the work. Lyontec® Chemicals, S. de R. L. de C.V. (Mexico), donated the lipid excipients (Transcutol® HP, Labrafil®, and Labrasol®) from Gattefossé® (USA) which used to obtain the SEDDS formulations, and Tween® 80, Propylene glycol®, and affinin standards were acquired from ChromaDex® Standards (USA).
Marrero-Morfa, D., Ibarra-Alvarado, C., Luna-Vázquez, F.J. et al. Self-microemulsifying system of an ethanolic extract of Heliopsis longipes root for enhanced solubility and release of affinin. AAPS Open 9, 17 (2023). https://doi.org/10.1186/s41120-023-00086-5
Read also our introduction article on Orally Disintegrating Tablets (ODTs) here:


