Based on Eudragit® encapsulated ionic polymer IR775@nido-carborane strategy: release, bioactivity and tumor cell imaging studies in simulated gastrointestinal environment
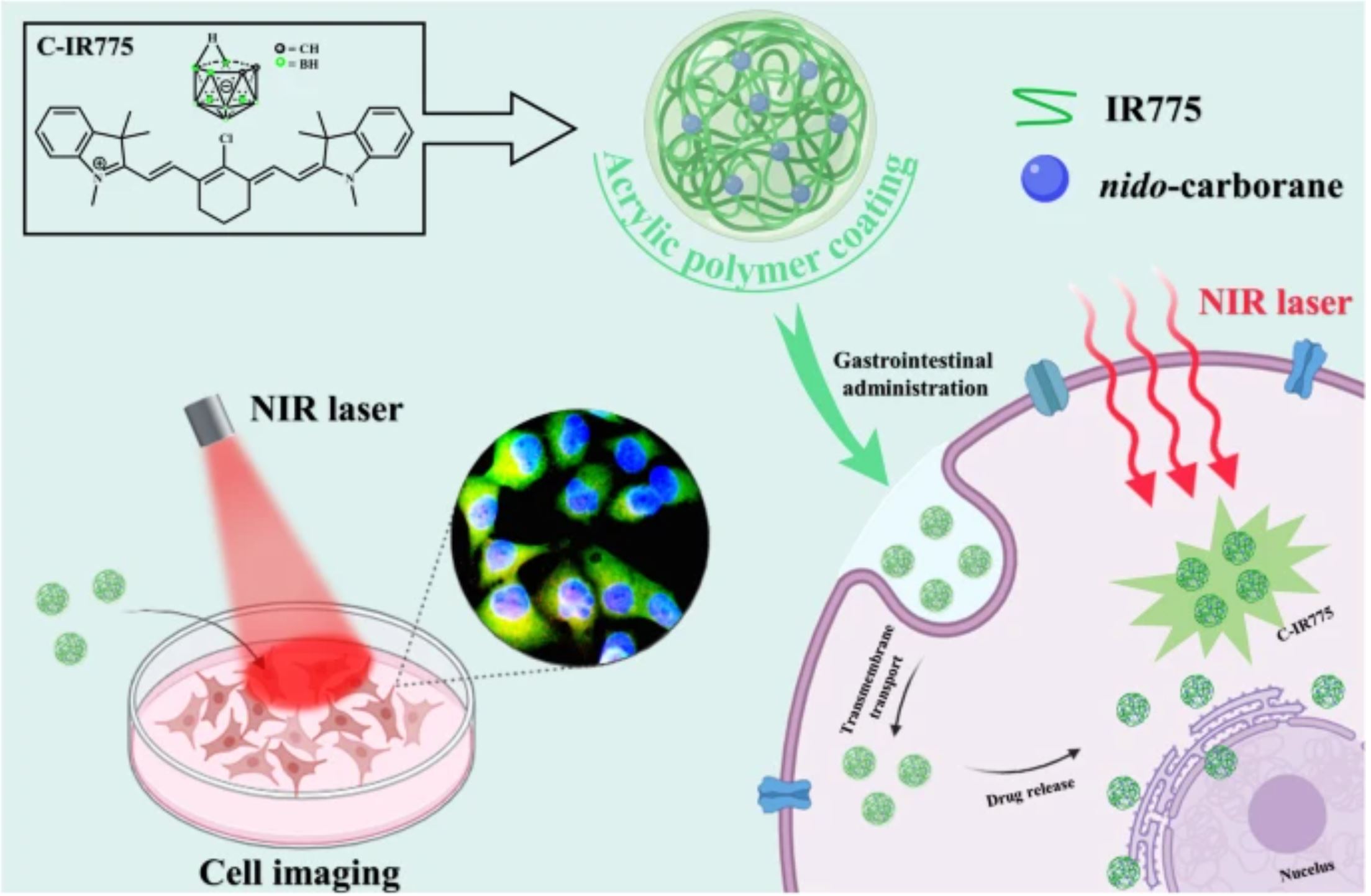
To enhance the bioavailability of carborane as a potential pharmacophore for BNCT, we labeled the modified o-carborane with the near-infrared dye IR775 and encapsulated it using two types of Eudragit® (pH-sensitive and osmotic). Consequently, four separate fluorescent complexes containing carborane were acquired. To confirm the nido-carborane presence within these complexes, the distinct peak at 2510 cm−1 was detected using infrared spectroscopy as a first step. The photophysical properties were observed in phosphate buffer with different pH.
The UV and fluorescence spectra of the four fluorescent complexes were very similar, with the maximum absorption wavelengths centered in the range of 773–789 nm and the emission wavelengths centered in the range of 796–811 nm. Subsequently, a stable and releasing complex L100-C-IR775 was screened through zeta potential testing and simulated release experiments in the gastrointestinal environment, and spherical shape was observed by transmission electron microscopy. AFM imaging showed a relatively smooth surface with a uniform distribution of protrusions. The L100-C-IR775 was then applied to tumor cell imaging, and it was observed that it could enter into three kinds of tumor cells, A549, HCT116 and HeLa, and distribute around the nucleus.
Finally, it was shown by a cell proliferation toxicity assay (CCK8) that the compound inhibited HeLa and PC-3 cells by 50 and 51% at concentrations up to 10 µg/mL. In conclusion, the carborane fluorescent complexes prepared in this paper have good biocompatibility and demonstrate toxicity toward tumor cell proliferation. Furthermore, they have the potential to serve as a carbon borane anti-tumor prodrug.
Read more here
Wang, S., Liu, Y., Zhou, M. et al. Based on Eudragit® encapsulated ionic polymer IR775@nido-carborane strategy: release, bioactivity and tumor cell imaging studies in simulated gastrointestinal environment. Macromol. Res. (2024). https://doi.org/10.1007/s13233-024-00250-0
See our next webinar:
“Rethinking the development of controlled release formulations and manufacturing processes”
Date: 30th of April, Time: 3:00 pm (Amsterdam, Berlin)


