The relationship between molecular-structural properties and functional-related characteristics of croscarmellose sodium produced by different manufacturers
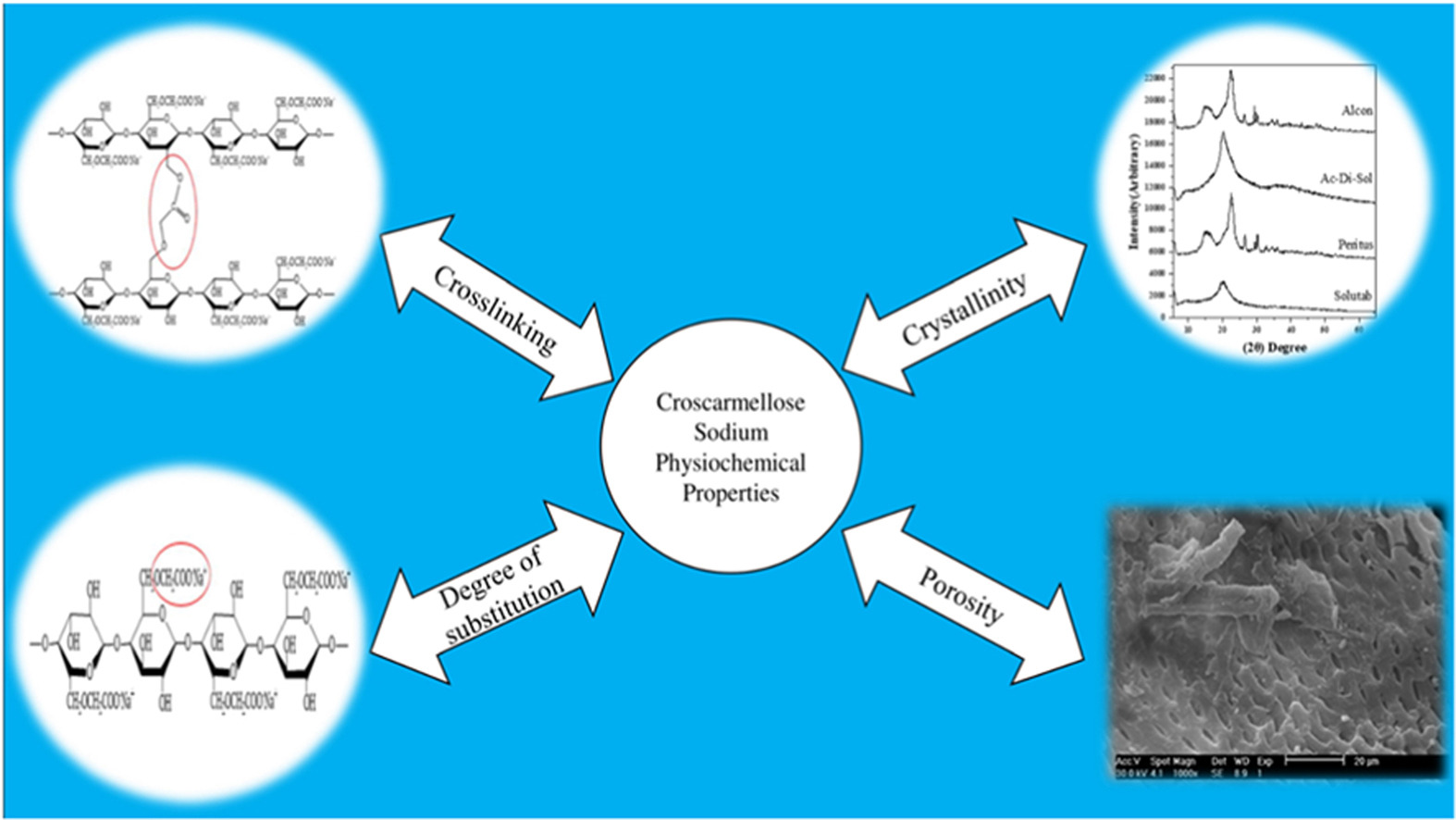
Croscarmellose sodium (CCS)is a superdisintegrant used in the formulation of pharmaceutical tablets. As with most other pharmaceutical ingredients, CCS is produced by various manufacturers. Our previous work has shown that products of different manufacturers can have varying properties and performance. The aim of this study was to find the underlying cause of this variation. CCS was obtained from four manufacturers. Different physical and molecular properties of the four samples of CCS were studied using tests such as SEM, BET, particle size analysis, XRD, DSC, TGA, settling volume, and wetting time. While swelling is the primary mechanism of action of CCS, the CCS samples studied could be divided into two groups of low and highly-swelling products based on the results obtained. The XRD results indicated that the high-swelling CCSs were primarily amorphous, while the low-swelling products were semi-crystalline. The other test results corroborated these conclusions. This ‘polymorphism’, which has not been reported before, was explained by possible variations in the manufacturing process of this superdisintegrant.
Introduction
A disintegrant is often included in the formulation of immediate-release tablets to facilitate their disintegration. Disintegration can be defined by the breakdown of the inter-particulate bonds formed during tablet compression. Therefore, disintegrants exert a force on the tablet structure greater than the forces between the particles, disrupting the bonds. This results in a significant increase in surface area and, consequently, enhancement of the dissolution of the active pharmaceutical ingredient. Traditionally, ordinary disintegrants, such as starch, were used. However, because of their often poor action, they have been replaced, to a large extent, by more efficient disintegrants or superdisintegrants. Croscarmellose sodium (CCS), a cross-linked carboxymethyl cellulose polymer, is an example of a superdisintegrant used in pharmaceutical solid dosage forms, most notably tablets.
Several mechanisms have been proposed to explain the action of disintegrants. However, water wicking, swelling, and strain recovery are considered to be the main ones. Swelling, possibly the main mechanism for tablet disintegration, is the omnidirectional expansion of particles that creates pressure, pushes adjacent particles apart, exerts stress on the entire system, and ultimately disintegrates the tablet [1,2].
Considering that a comprehensive understanding of the relationship between the characteristics of superdisintegrants and their functionality is still elusive [3], previously, the authors studied the pharmacopeial characteristics and the performance of four CCS products [4]. It was found that the samples from two manufacturers (Alcon and Peritus) had a very limited swelling ability, while the other two brands (FMC and Roquette) swelled extensively. The highly swelling CCSs resulted in tablets with superior dissolution profiles.
Although previous research on croscarmellose sodium (CCS) has established physicochemical differences between products of different manufacturers [5], possible underlying structural differences and their relationship to the manufacturing process of CCS have not been investigated. It is hypothesized that possible variations in the performance of CCS samples produced by different manufacturers are the result of sub-particle structural differences between the different brands as well as particle-level properties, such as particle size, both of which are under the influence of CCS’s production procedures.
Therefore, unlike previous research on the subject, the current study aimed to investigate the four CCS samples’ molecular and supramolecular structures to determine the possible reason(s) for the observed functional differences. To reach this goal, while considering the influence of CCS manufacturing procedures on the final product, instrumental analyses as well as several physicochemical tests were performed on the four CCS samples.
Materials
The croscarmellose samples were obtained from four manufacturers: Ac–Di–Sol® (FMC International, Ireland, Lot # T1618C), Solutab® (Roquette, Brazil, Lot # 195008440), “Alcon” (Alcon Biosciences Pvt Ltd, India, Lot # PR/P/CR-0145/19), “Peritus” (Peritus International, India, Lot # PR/P/CR-0291/18).
Read more
Arash Yavari, Mostafa Saffary, Ali Nokhodchi, Seyed Kazem Sadjady, The relationship between molecular-structural properties and functional-related characteristics of croscarmellose sodium produced by different manufacturers, Journal of Drug Delivery Science and Technology, 2023, 104863, ISSN 1773-2247,
https://doi.org/10.1016/j.jddst.2023.104863.