Tableting properties of freeze-dried trehalose: physico-chemical and mechanical investigation
Abstract
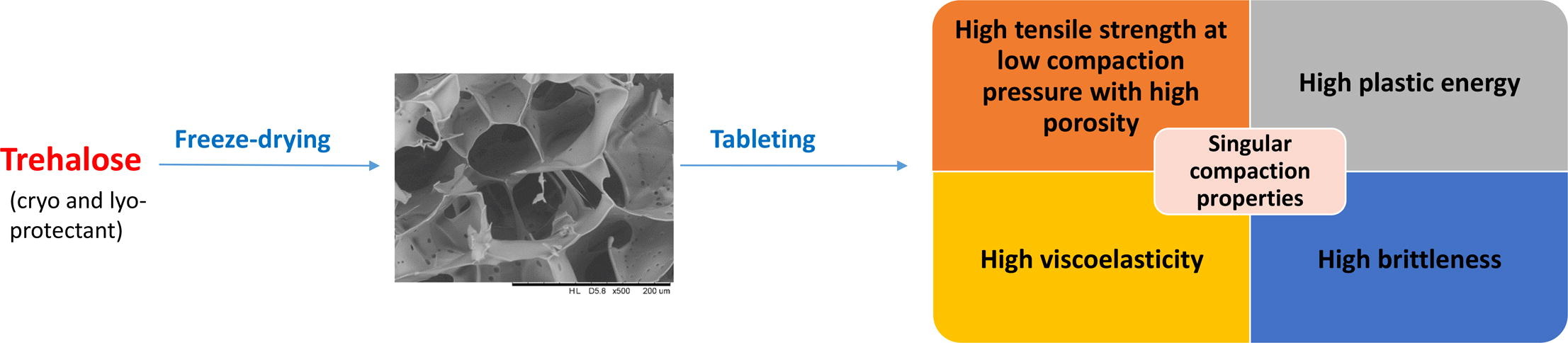
Freeze-drying of biopharmaceutical products is the method of choice in order to improve their stability and storage conditions. Such Freeze-dried products are usually intended for parenteral route administration. However, many biopharmaceutical materials administered by parenteral route are used to treat local diseases particularly in the gastro-intestinal tract. Therefore, many studies concentrate nowadays their effort on developing alternative dosage forms to deliver biopharmaceutical molecules by the oral route. Tablets are the most popular solid pharmaceutical dosage form used for oral administration since they present many advantages, but poor informations are available on the possibility of tableting freeze-dried powders. In this study, we evaluate the compaction behavior of freeze-dried trehalose powder since trehalose is one of the most used cryo and lyoprotectant for the lyophilisation of biopharmaceutical entities. Results show that freeze-dried trehalose powder can be tableted while remaining amorphous and the obtained compacts present very specific properties in terms of compressibility, tabletability, brittleness and viscoelasticity compared to the crystalline trehalose and compared to classical pharmaceutical excipients.
Introduction
Biopharmaceutical molecules represent nowadays a major therapeutic class and their use gained lots of attention in the last decades since they cover a large panel of therapeutic indications such as cancer, auto-immune disorders, neurological diseases or metabolic disorders (Kesik-Brodacka, 2018). Indeed, the COVID 19 pandemic has boosted the biopharmaceutical market with the development of vaccines and monoclonal antibodies against SARS CoV2 virus (Jaworski, 2021).Table 1.Table 2.
Biopharmaceutical active substances such as therapeutic proteins, vaccines or cell and gene therapies are usually obtained and prepared in liquid dosage forms intended for parenteral administration. However, one major challenge in using biopharmaceutical molecules refers to their complex and sensitive macromolecular structure making them prone to physicochemical instabilities such as hydrolysis, deamidation, aggregation or degradation when formulated as aqueous solution (Butreddy et al., 2021). Therefore, dry solid state may be preferred in order to improve their stability. Amongst the different drying strategies, freeze-drying is the method of choice. Freeze-dried drug delivery systems present better biological stability and allow for better shipping, storage and handling conditions (Liu and Zhou, 2021, Sharma et al., 2021). Process control of freeze-drying implies both the choice of formulation and process parameters and is essential to preserve biopharmaceutical molecules and to achieve the required product quality attributes (Hsein et al., 2022). Formulation strategies mainly consist in adding cryo and lyoprotectors such as sucrose and trehalose, surfactants such as polysorbate 20 or 80, polyols such as mannitol or glycine, or choosing buffer salts to adjust the pH (Bjelošević et al., 2020). Amongst these excipients, the non-reducing sugar trehalose is the most widely used as stabilizer both in commercial biopharmaceutical products and in research papers. The stabilization mechanisms of trehalose during freeze-drying is generally explained by the water replacement theory and by the formation of amorphous materiallimiting molecular mobility (Ohtake and Wang, 2011, Olsson et al., 2016).
Moreover, obtaining dry products opens the way to the development of other dosage forms than those dedicated to the parenteral administration. Then, developing oral route for biopharmaceutics has been the goal of many studies in the last decades although it still remains highly challenging since the absorption of such hydrophilic and high molecular weight molecules is very limited (Haddadzadegan et al., 2022, Homayun et al., 2019, Madani et al., 2020, Olorunsola et al., 2023, Vass et al., 2019). However, the oral administration of these molecules for the local treatment of some gastrointestinal pathologies may be an interesting option. Nevertheless, this administration will require additional formulation steps to protect the biomolecules from degradation by gastric acidity and by the gastrointestinal tract proteases (Choonara et al., 2014, Homayun et al., 2019). For oral administration, tablets are the most popular solid pharmaceutical dosage form since they present many advantages such as being easy to take and improving patient compliance. Other advantages from industrial standpoint, are the low cost of production and above all, the absence of cold chain requirements and sterility issues (Zhong et al., 2018). However, producing a tablet by powder compaction from a freeze-dried (FD) solid requires additional knowledge and very little information is available about the tableting behavior of these powders. Therefore, the aim of this study is to investigate by a systematic approach, the compaction behavior of FD trehalose and finally, to conclude on a possible use in tablet formulations. Then, a detailed analysis of the physico-chemical and mechanical properties of FD trehalose tablets is carried out and discussed in comparison with crystalline trehalose and traditional pharmaceutical excipients commonly used in direct compression processes.
Read more here
Materials
Trehalose dihydrate used for freeze-drying was generously given by DFE Pharma (Goch, Germany) and the one used for direct compression was generously given by Nagase (Kyoto, Japan). Three other excipients were used to make tablets: anhydrous calcium phosphate (ACP) (Anhydrous Emcompress®, JRS Pharma, Rosenberg, Germany), granulated lactose monohydrate (GLac) (Tablettose® 80, Meggle, Wasserburg, Germany) and microcrystalline cellulose (MCC) (Vivapur® 12, JRS Pharma Rosenberg, Germany).
Hassana HSEIN, Charbel MADI, Vincent MAZEL, Pierre TCHORELOFF, Virginie BUSIGNIES, Tableting properties of freeze-dried trehalose: physico-chemical and mechanical investigation, International Journal of Pharmaceutics, 2023, 123598, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123598.
Read also more article about freeze drying:
- Stability study of spray freeze-dried insulin dry powder formulations used for nose-to-brain delivery
- A mucoadhesive patch loaded with freeze-dried liposomes for the local treatment of oral tumors
- Maintaining supersaturation generation and protein integrity of amorphous curcumin-albumin nanoplex during storage by freeze drying with trehalose