Stability study of spray freeze-dried insulin dry powder formulations used for nose-to-brain delivery
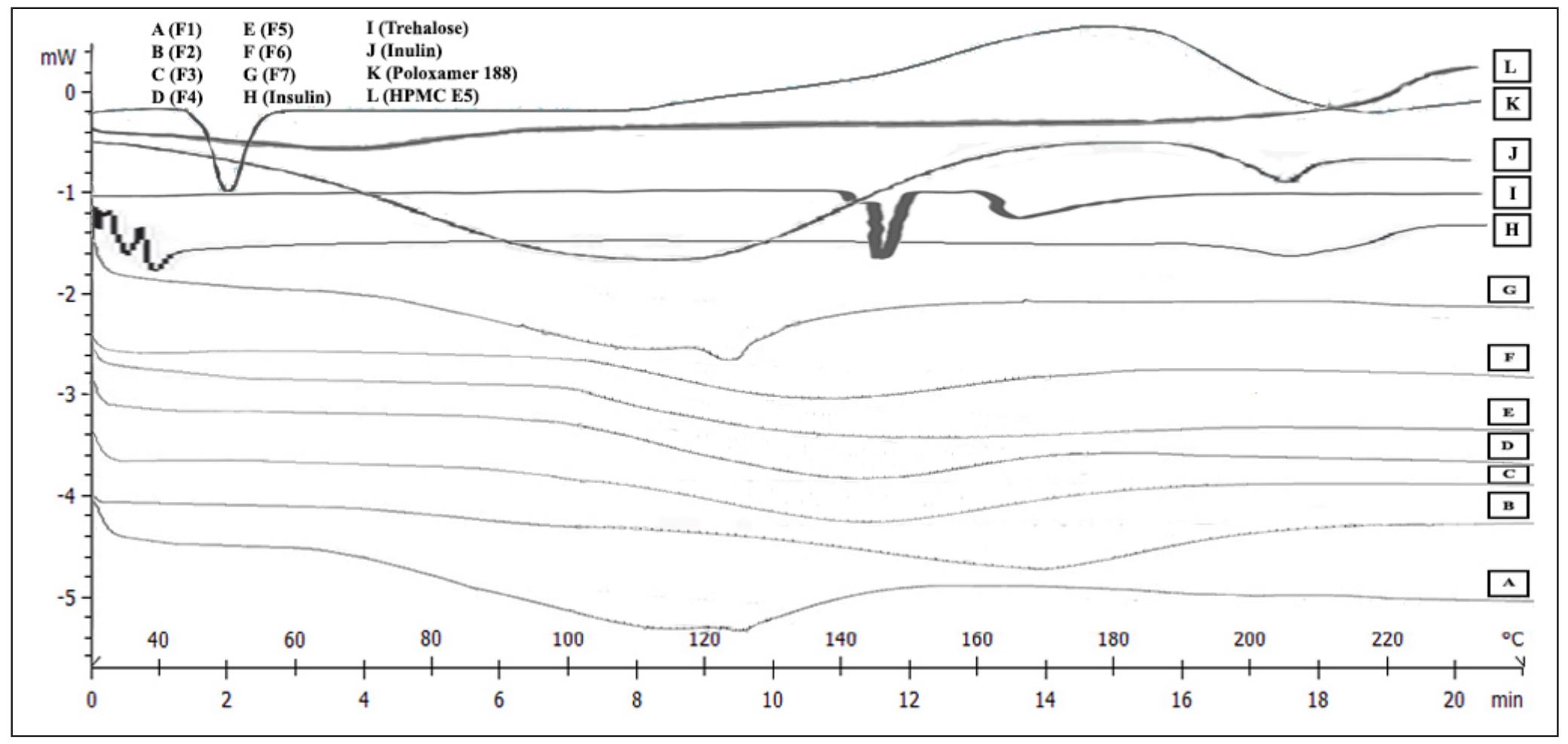
Insulin is classified as a cold chain product due to being a peptide hormone with stability issues in the liquid preparation. Therefore, insulin was developed into the dry powder form to improve the stability and application for nose-to-brain delivery in Alzheimer’s disease treatment. Insulin was physically engineered through the addition of sugar stabilizers with seven different weight ratios of trehalose to inulin, labeled as F1–F7, and prepared using the spray freeze-drying (SFD) method.
The obtained SFD insulin dry powders (IDP) were characterized physically and chemically. In addition, the long-term stability study was conducted at 25°C and 40°C for 6 months, whereas the accelerated stability study was examined at 40°C, 50°C, and 60°C for 1 month. This study aims to obtain the most stable IDP formulation. The results show that IDP F3, composed of trehalose and inulin 1:1 w/w, was the superior formula.
Moreover, IDP F3 exhibited spherical shapes with rough surfaces, amorphous crystallinity, and high insulin content of 100%. Furthermore, IDP F3 indicated the proper stability for 6 months, including insulin content, transition glass temperature (Tg), and moisture content. According to stability study results, the k25 value, half-life, and shelf life of IDP F3 were (1.77 ± 0.06)×10−2 week−1, 39.17 ± 1.34 weeks, and 5.93 ± 0.20 weeks, respectively.
Download the full article as PDF here Stability study of spray freeze-dried insulin dry powder formulations used for nose-to-brain delivery
or read it here
Materials
Recombinant human insulin, trehalose, inulin, hydroxypropyl methylcellulose(HPMC) E5, and Poloxamer 188 of pharmaceutical grade were purchased from Sigma Aldrich (St. Louis, MO). The water for injection was purchased from Ikapharmindo Putramas (Jakarta, Indonesia). Whatman nylon membrane filters 0.2 μm was obtained from GF Healthcare Life Sciences (Germany). Moreover, KH2PO4, NaOH, HCl (proanalysis), acetonitrile, and methanol of gradient grade for liquid chromatography, and trifluoroacetic acid for spectroscopy were purchased from Merck (Darmstadt, Germany).
Stability study of spray freeze-dried insulin dry powder formulations used for nose-to-brain delivery, Cynthia Marisca Muntu, Christina Avanti, Hayun Hayun, Silvia Surini, Published: Oct 04, 2023, DOI: 10.7324/JAPS.2023.148983
See the webinar:
“Rational Selection of Cyclodextrins for the Solubilization of Poorly Soluble Oral Drugs”, 8. November 2023:
Get more information & register here for free:


