Process development and quality attributes for the freeze-drying process in pharmaceuticals, biopharmaceuticals and nanomedicine delivery: a state-of-the-art review
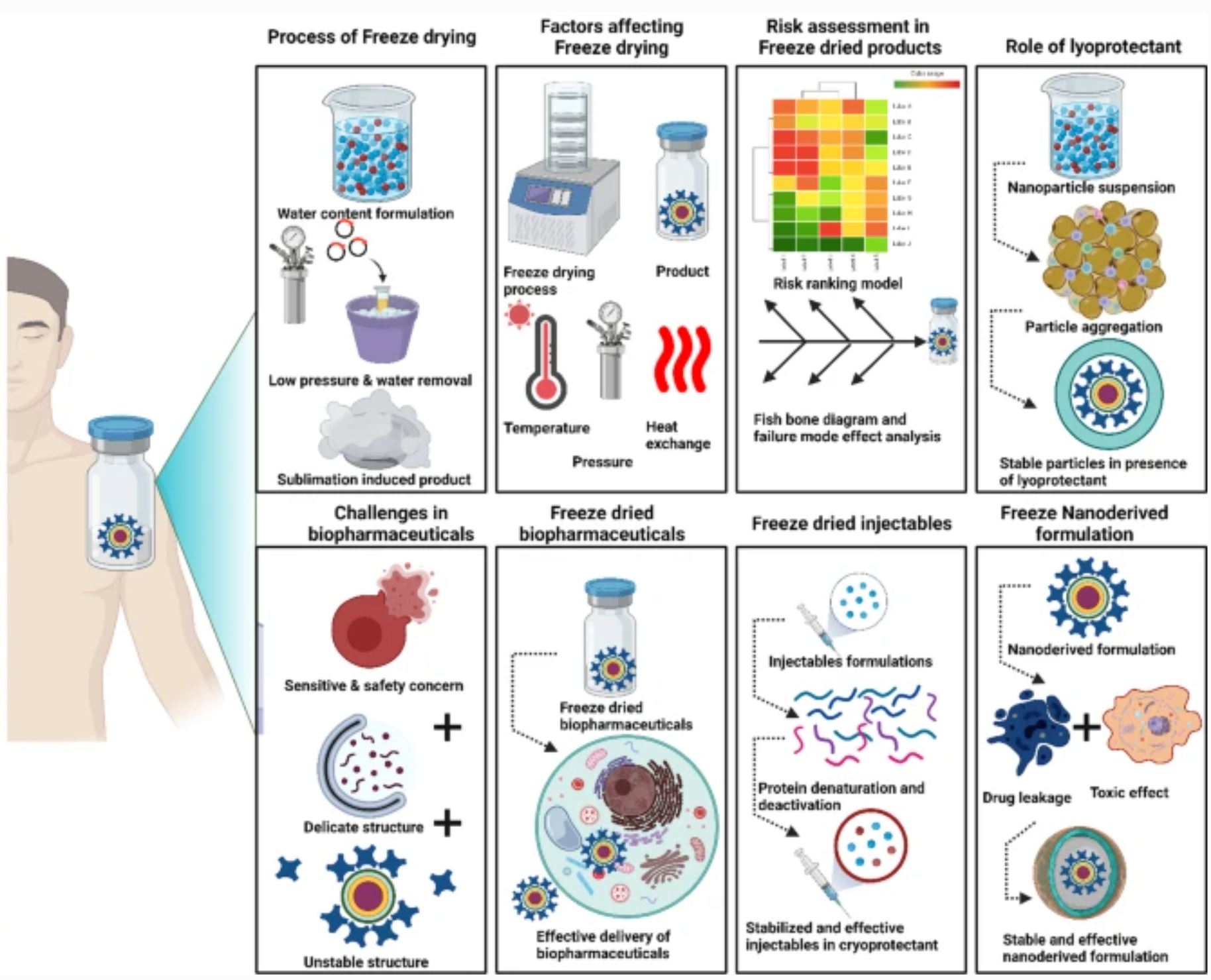
Abstract
Background
Process intensification is a major hurdle in pharmaceutical process scale-up. Solvent removal strategies have limited the effectiveness of the overall stability of pharmaceutical formulations. The main aim of present review article is to focus on the use of the freeze-drying process in pharmaceuticals, biopharmaceuticals and nanoderived therapeutics and their translation into commercial viable products. Unwavering efforts of scientists in the process intensification of lyophilization promote unique features of products for commercialization. Regulatory agencies are promoting the utilization of a quality-by-design approach to improve product characteristics. Among 300 FDA-approved pharmaceutical industries, 50% of products are freeze-dried. The freeze-drying process is costlier and requires more time than other drying methodologies. Unstable pharmaceutical dispersions and solutions can be preferably stabilized by using the freeze-drying method.
Main text
This review highlights the utilization of critical quality attributes and process parameters for the freeze-drying process, which helps to improve the integrity and stability of the formulation. The quality-by-design approach possibly cuts the cost of the process and saves money, time, and laborious work. The present review focuses preliminarily on the applications of freeze-drying in the development of biopharmaceuticals, including vaccines, proteins and peptides, and injectable products. In addition, a separate section demonstrating the potential of freeze-drying in nanoderived therapeutics has been illustrated briefly. The present clinical scenario of freeze-dried pharmaceuticals and biopharmaceuticals has also been described in later sections of the review.
Conclusions
This review underscores the value of integrating Quality by Design into the development of lyophilization processes for pharmaceutical and biopharmaceutical products. By identifying critical process parameters, delineating a design space, and leveraging advanced monitoring techniques, manufacturers can effectively address the intricacies of lyophilization. This approach empowers them to produce stable, superior quality products with confidence and consistency.
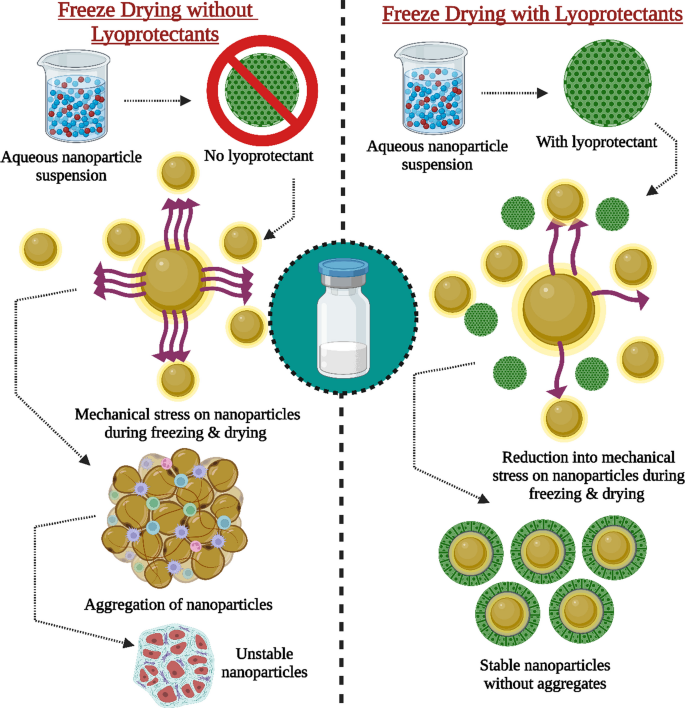
Table 3 Diferent types of excipients commonly used as lyoprotectants in freeze-drying of nanoformulations
| Excipients as lyoprotectants | Molecular weight (g/mol or kDa) | Optimum concentration of lyoprotectants (% w/w or mM) | Glass transition temperature (Tg, ℃) | Functions |
|---|---|---|---|---|
| Bulking agents | ||||
| Glycine | 75.07 | 10 | −70 to −90 ℃ | Provide bulk to the formula- |
| Hydroxyethyl starch | 670 kDa | 12.5 | 44 ℃ | tion, particularly if the product |
| Lactose | 342.3 | 1–2 | 101 ℃ | concentration to freeze-dry seems to be very low |
| Mannitol | 182.18 | 6 | 13 ℃ | |
| Sucrose | 342.3 | 2.5 | −46 ℃ | |
| Trehalose | 378.33 | 20 | 106 ℃ | |
| Buffers | ||||
| Citrate | 192.12 | NA | 105 ℃ | Adjust and maintain pH |
| Histidine | 155.15 | 3.5 mM | −32 ℃ | changes during freezing |
| Phosphate | 94.97 | 20 mM | NA | |
| Tris HCl | 121.14 | NA | −81 ℃ | |
| Stabilizers | ||||
| Alanine | 89.09 | 1–5 | −10 to 35 ℃ | Ofer protection from freezing |
| Dextran | 40–70 kDa | 2 | 68.2℃ | and drying stresses dur- |
| Glycerol | 92.09 | NA | NA | ing the freeze-drying process |
| Polyethylene glycol 400 | 380–420 g/mol | 25–35 | −63 ℃ | |
| Polyvinyl pyrrolidone | NA | 7.5 | NA | |
| Sodium chloride | 58.44 | 5 M | NA | |
| Tonicity adjuster | ||||
| Glycine | 75.07 | 10 | −70 to −90 ℃ | Produce an isotonic solution |
| Glycerol | 92.09 | NA | NA | while controlling osmotic |
| Mannitol | 182.18 | 6 | 13 ℃ | pressure |
| Sucrose | 342.3 | 2.5 | −46 ℃ | |
| Sodium chloride | 58.44 | 5 M | NA | |
| Collapse temperature modifers | ||||
| Hydroxypropyl-β-cyclodextrin 1 | 1387 | 5–10 | 418 ℃ | Raise the product’s collapse temperature to attain optimal drying temperatures |
Download the full article as PDF here Process development and quality attributes for the freeze-drying process in pharmaceuticals, biopharmaceuticals and nanomedicine delivery: a state-of-the-art review
or read it here
Pardeshi, S.R., Deshmukh, N.S., Telange, D.R. et al. Process development and quality attributes for the freeze-drying process in pharmaceuticals, biopharmaceuticals and nanomedicine delivery: a state-of-the-art review. Futur J Pharm Sci 9, 99 (2023). https://doi.org/10.1186/s43094-023-00551-8

