Effect of binder type on physical and in vitro properties of high dose inosine acedoben dimepranol tablets
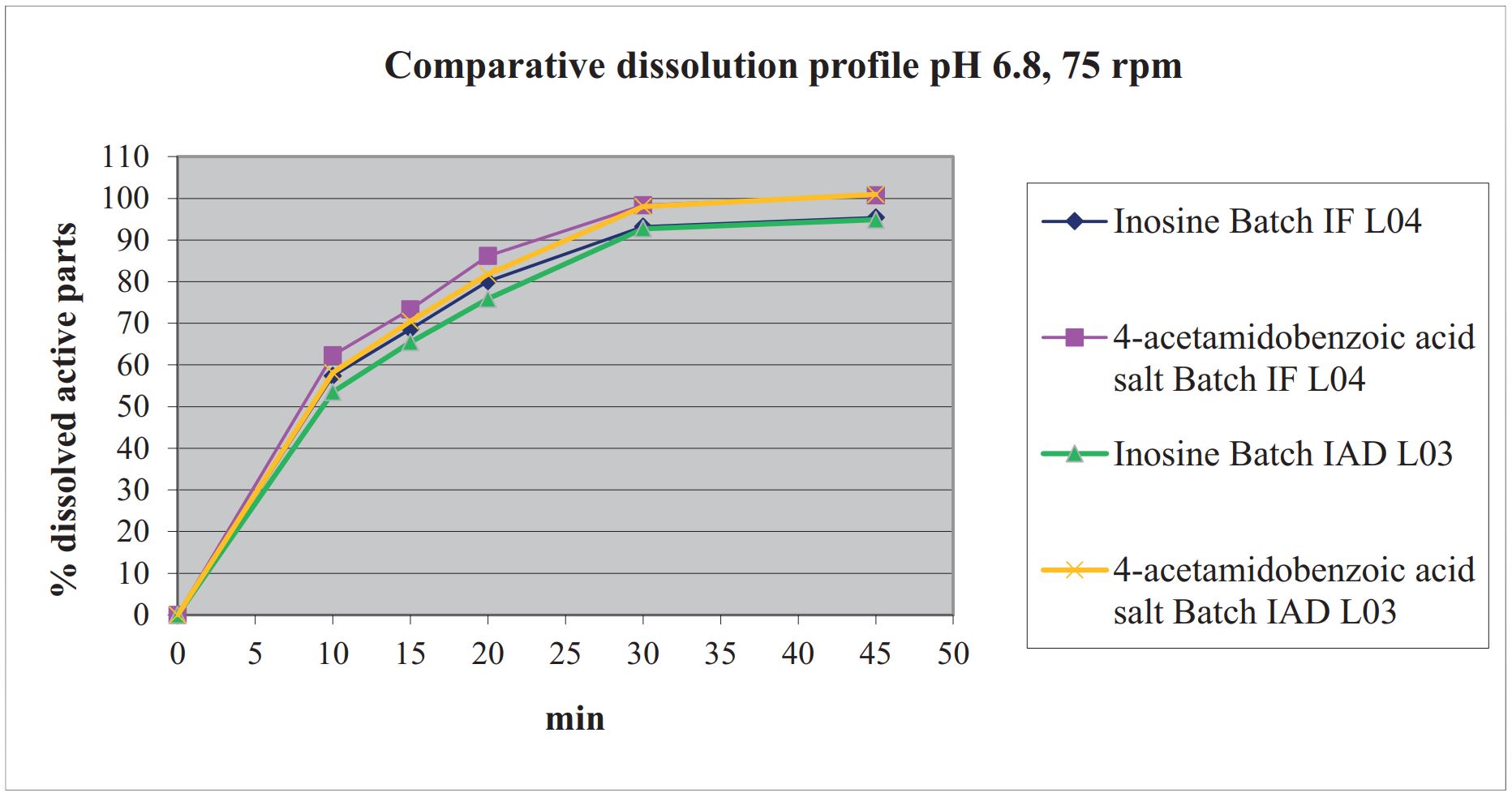
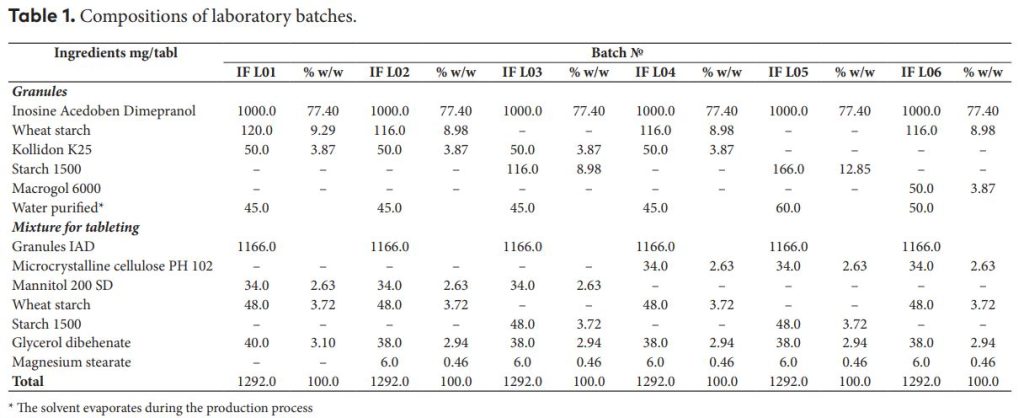
The present work was conducted with the aim to formulate and evaluate the immediate release of 1000 mg tablets containing Inosine Acedoben Dimepranol (IAD). All the samples of the tablets containing 1000 mg IAD were prepared by conventional wet granulation method. Various type of binders like povidone, wheat starch, pregelatinized starch (starch 1500TM , Colorcon), microcrystalline cellulose and macrogol 6000 were used to prepare some series of tablets. Granules were evaluated for pre-compression parameters and tablets were evaluated for post- compression parameters. The composition batch № IAD1000 L04 was found to be the best for producing IAD 1000 mg tablets. The optimal lubricant system is glycerol dibehenate at a concentration of 2.94% plus magnesium stearate at a concentration of 0.46%. Optimized formulation was evaluated for in-vitro dissolution test. Stability studies were performed for the selected composition.
Introduction
The active substance Inosine Acedoben Dimepranol (INN) (IAD), also known as Inosine pranobex and Methisoprinol has been proven to positively impact the host’s immune system, by enhancing T-cell lymphocyte proliferation and activity of natural killer cells, increasing levels of proinflammatory cytokines, and thereby restoring deficient responses in immunosuppressed patients. At the same time, it has been shown that it can affect viral RNA levels and hence inhibit growth of several viruses. Due to its immunomodulatory and antiviral properties as well as its safety profile, it has been widely used against viral infections and diseases among which subacute sclerosis panencephalitis, herpes simplex virus, human papilloma virus, human immunodeficiency virus, influenza and acute respiratory infections (Sliva et al. 2019).
The drug was developed by Newport Pharmaceuticals in the 1970s and distributed in more than 80 countries under
the trademarks IsoprinosineTM, InmunovirTM and ViruxanTM (owned by Newport) for the treatment of viral infections.
According to the Summary of product characteristics of the product IsoprinosineTM 500 mg tablets (Bulgarian drug agency 2022), the dosage is as follows: for adults and elderly patients, the recommended daily dose is 50 mg/kg of body weight (1 tablet per 10 kg), usually 3 g/day to no more than 4 g inosine acedoben dimepranol daily, administered orally in 3–4 equally divided doses during the day.
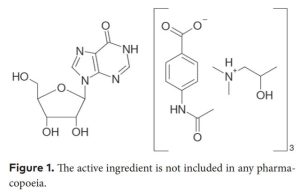
Due to its potent immunomodulatory properties, IAD has been intensively evaluated for its potential as a COVID-19 medication (Beran et al. 2021). For the treatment of COVID-19 it is recommended to use the maximum dosage of 4×2 tabl. (4×1000 mg = 4 g/ day). The active substance is a compound (complex) of inosine (1,9-dihydro-9-Dribofurasonyl-6H-purin-6-one) and a salt of 4-acetamidobenzoic acid with N, N dimethylamino-2-propanol in a molar ratio of 1: 3. It is a crystalline powder with a white to cream color and a characteristic odor, freely soluble in water, sparingly soluble in methanol, acetone, ethanol (Gordon P 1974):
In the first publication (Kafedjiiski K 2022) it is concluded: „Based on the results of the study, an optimal composition and process for the production of IAD 500 mg tablets with immediate release of the active substance is proposed” (p. 324). Since in most indications of IAD 500 mg tablet a single dose of 1000 mg is prescribed, the development of 1000 mg tablet is undertaken, which is more convenient for the patient. The shape of the tablet should be oblong, which makes it easier for the patient to swallow it. In the pharmaceutical practice, there are a number of examples of popular active substances with a dose of 1000 mg or more in the form of tablets, such as paracetamol, piracetam, metformin, etc.
The 1000 mg dose of the as the dosage form Isoprinosine powder for oral solution in a sachet was introduced on the
market in Bulgaria in 2020. Children and adults who have trouble swallowing tablets or capsules may find powders for
oral solution more acceptable. Dissolution rate of oral powders containing water-soluble drugs is generally faster than
tablets or capsules, in which disintegration of the tablet or the capsule shell is required prior to dissolution. A disadvantage of powder forms is that they are bulky and inconvenient to be carried. A glass of water and a spoon are needed to dissolve the product before administration. Masking the unpleasant taste of the active substance can be a problem with this type of dosage form. The data of IMS Bulgaria for the annual sales in 2021 of Isoprinosine products are as follows: tablets 500 mg × 50 – 172 413 packages (5 849 313 BGN), powder for oral solution × 24 – 5 008 packages (183 472 BGN). Therefore, the share of tablets in sales is about 97% and it is the most preferred form by patients.
Tablets 1000 mg IAD have already been registered in Poland under the trade name Neosine forte – Aflofarm
Farmacja Polska Sp. z o.o. in 2019. The dosage is the same: the recommended dose is 50 mg/kg body weight daily
(usually 1000 mg, i.e. 1 tablet administered 3 to 4 times a day). The maximum dose is 4.000 mg daily. In the Summary of product characteristics of Neosine forte tablets, the following excipients are described: wheat starch, mannitol, povidone and magnesium stearate. The literature review shows there are no articles on the pharmaceutical development of IAD tablets. The approach to develop a higher dose of a solid dosage form i.e. tablets in pharmaceutical and regulatory practice is usually based on the principle of proportionality. Preliminary experiments have shown this principle is not fully applicable in the case of the IAD substance. On the other hand, in the development of a 500 mg tablet, the solvent ethanol 96%: water = 1: 1 for granulation is used, and in industry only water is preferred as a solvent due to ecological and explosiveness related concerns. Therefore, the aim of this work is to evaluate and compare the effect of some commonly used binders and develop a 1000 mg IAD tablet with immediate release of the active substance by using only water solvent for the granulation process.
Download the full article as PDF here Effect of binder type on physical and in vitro properties of a high dose inosine acedoben dimepranol tablets
Materials
Inosine Acedoben Dimepranol (ABC Farmaceutici S.p.A., Italy), wheat starch (Roquette), povidone K25 (Kollidon 25, 30 BTC Europe GmbH/ BASF), pregelatinized starch (starch 1500, Colorcon), Macrogol 6000 (Clariant), microcrystalline cellulose PH 102 (Vivapur, JRS Pharma), mannitol (Pearlitol 200 SD, Roquette), glycerol dibehenate (Compritol 888 ATO, GATTEFOSSE), magnesium stearate (Peter Greven); all excipients meet the requirements of Ph. Eur.
Kafedjiiski K (2022) Effect of binder type on physical and in vitro properties of high dose inosine acedoben dimepranol tablets. Pharmacia 69(4): 947–953. https://doi.org/10.3897/pharmacia.69.e89410

