Development of a novel squalene/a-tocopherol-based self-emulsified nanoemulsion incorporating Leishmania peptides for induction of antigen – specific immune responses
Abstract
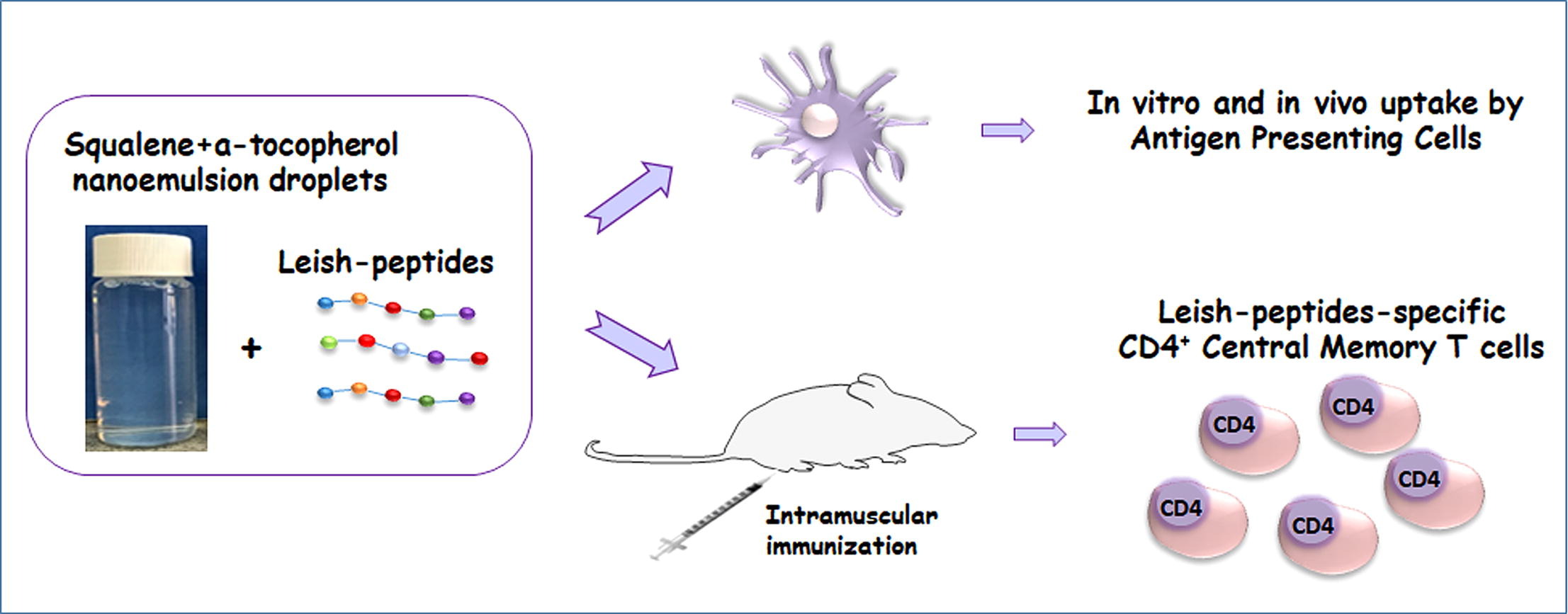
Vaccination has emerged as the most effective strategy to confront infectious diseases, among which is leishmaniasis, that threat public health. Despite laborious efforts there is still no vaccine for humans to confront leishmaniasis. Multi-epitope protein/peptide vaccines present a number of advantages, however their use along with appropriate adjuvants that may also act as antigen carriers is considered essential to overcome subunit vaccines’ low immunogenicity. In the present study, a stable self-emulsified nanoemulsion was developed and double-adjuvanted with squalene and a-tocopherol. The prepared nanoemulsion droplets exhibited low cytotoxicity in a certain range of concentrations, while they were efficiently taken up by macrophages and dendritic cells in vitro as well as in vivo in secondary lymphoid organs. To further characterize nanoformulation’s potent antigen delivery capability, three multi-epitope Leishmania peptides were incorporated into the nanoemulsion. Peptide encapsulation resulted in dendritic cells’ functional differentiation characterized by elevated levels of maturation markers and intracellular cytokine production. Intramuscular administration of the nanoemulsion incorporating Leishmania peptides induced antigen-specific spleen cell proliferation as well as elicitation of CD4+ central memory cells, supporting the potential of the developed nanoformulation to successfully act also as an antigen delivery vehicle and thus encouraging further preclinical studies on its vaccine candidate potency.
Introduction
Vaccination is considered as one of the most effective strategies against infectious diseases, a major threat for public health worldwide. The development of effective vaccines against intracellular pathogens that require the induction of cellular immune responses has been proven a difficult challenge. One such example is leishmaniasis, a group of diseases caused by the obligate intracellular parasite Leishmania spp., where despite laborious efforts there is no available vaccine against human disease (Mann et al., 2021). Vaccination strategies based on subunit or multi-epitope peptide vaccines have gained the attention of researchers due to certain advantages such as absence of infectious agents, stability, specificity and large-scale production at low cost (De Brito et al., 2018). However, protein or peptide vaccines often fail to induce strong and effective immune responses due to their low immunogenicity (De Brito et al., 2018, Skeiky et al., 2002). Thus, the use of appropriate adjuvants acting as delivery systems or directly acting on receptors of innate immune cells to stimulate them has been proposed to overcome peptide vaccines’ low immunogenicity (Askarizadeh et al., 2020, Coffman et al., 2010).
Vaccine adjuvants are a diverse family, comprising several naturally occurring or synthetic materials that boost the immunological effect of the antigen. Among these adjuvants, squalene oil-based emulsion adjuvants, such as MF59 and AS03, have shown significant enhancement of immune responses to influenza vaccine antigens even with reduced antigen dose as well as reduced immunizations number (Vogel et al., 2009, Wilkins et al., 2017). AS03 is a squalene oil-based oil-in-water emulsion adjuvant containing a-tocopherol as an additional immunostimulant able to induce more robust immune responses as compared to adjuvants containing only squalene oil (Garcon et al., 2012, Morel et al., 2011, Yam et al., 2015). These emulsion adjuvants are typically produced via complex manufacturing and high energy processes (e.g., microfluidization, high pressure homogenization) using challenging equipment with high maintenance requirements (Lodaya et al., 2019, O’Hagan et al., 2021), thus limiting their production and distribution only to affluent countries. Accordingly, there is an urgent need for the development of emulsion adjuvants based on simpler, cost-effective methods (Lodaya et al., 2019, Rappuoli and Dormitzer, 2012).
Self-nanoemulsifying drug delivery systems (SNEDDS) are isotropic mixtures of oil, surfactant and co-surfactant freely forming oil-in-water (o/w) nanoemulsions of extremely small droplet diameter (e.g., <50 nm) upon mixing with an aqueous phase (e.g., gastrointestinal fluids). SNEDDS have more advantages than other lipid carriers, such as exceptional storage stability, easy, fast and efficient production on a large scale (Kassem et al., 2016). SNEDDS are typically used for the enhancement of the oral bioavailability of hydrophobic drugs (e.g., cyclosporine A) for which there exist marketed formulations such as Sandimmune® and Neoral® (Novartis, Basel, Switzerland) (Karamanidou et al., 2016). However, several recent studies have demonstrated that SNEDDS can be used for the delivery of hydrophilic biomolecules (e.g., therapeutic peptides) via the oral administration route (Karamanidou et al., 2015, Mahmood and Bernkop-Schnurch, 2019, Menzel and Bernkop-Schnurch, 2018). Additionally, Lupo and co-workers developed self-emulsifying drug delivery systems (SEDDS) in which they encapsulated bovine serum albumin (BSA) model antigen through hydrophobic ion pairing along with monophosphoryl lipid A (MPLA) or squalene adjuvant. Importantly, the developed formulations were found to induce both systemic and mucosal antigen-specific immunity after being orally administered to mice indicating not only their adjuvanticity but also their potential to be used as antigen carriers (Lupo et al., 2019).
More recently, the self-emulsification process was used to develop emulsion adjuvants similar in composition with MF59 and AS03, which were shown to have an adjuvant effect (Lodaya et al., 2019, Shah et al., 2015). Squalene-based self-emulsified emulsion adjuvants have been proven effective in eliciting antigen-specific antibodies against Mycoplasma hyopneumoniae and enterovirus (Bastola et al., 2019, Chae et al., 2022). Additionally, two experimental self-emulsified adjuvant systems containing both squalene oil and a-tocopherol were shown to induce humoral and cellular immune responses similar to AS03 despite the fact that the a-tocopherol content was lower (Lodaya et al., 2019). The essence of this process is the efficient, low-energy consuming scaled up production of adjuvanted vaccines in established facilities even in developing countries.
In the present study, a novel extremely stable (> 20 weeks) oil-in-water (o/w) nanoemulsion, doubly adjuvanted with squalene oil and a-tocopherol (ST-SNEDDS) and exhibiting low droplet diameter (< 30 nm) was developed based on the simple, easily scalable SNEDDS technology to be used for the efficient delivery of antigenic peptides via the s.c. and/or i.m. administration routes. The ST-SNEDDS’ physicochemical characteristics, biocompatibility and adjuvanticity were assessed in vitro, followed by studies regarding their in vivo tissue distribution and induction of immune cells infiltration in lymphoid organs after injection. Novel, in silico designed multi-epitope peptides derived from Leishmania proteome were subsequently incorporated in the nanodroplets through hydrophobic ion pairing with an anionic phospholipid. Their effect on DCs functional differentiation in vitro, as well as their potency to induce antigen-specific cellular immune responses in vivo were also assessed. It should be noted that, to our knowledge, this study is the first successful attempt to develop a self-emulsified nanoemulsion, doubly adjuvanted with squalene and a-tocopherol, incorporating multi-epitope antigenic peptides and to evaluate its potential as vaccine delivery vehicle using intramuscular immunization protocol in an experimental murine BALB/c model.
Read more here
Materials
1,2-Dimyristoyl-sn-glycero-3-phospho-rac-(1-glycerol) sodium salt (≥99%), macrogol (15)-hydroxystearate, polyethylene glycol (15)-hydroxystearate (Kolliphor® HS15), squalene (≥98%, liquid), (±)-α-tocopherol (tested according to Ph. Eur.), N,N-dimethylformamide (DMF) (for molecular biology ≥99%), phosphate buffered saline (PBS, 10x, pH 7.4), concanavalin A (ConA) and lipopolysaccharide (LPS) were purchased from Sigma-Aldrich (Vienna, Austria).
Preparation and characterization of SNEDDS
Nanoemulsions were successfully formed applying various oil/surfactant/co-surfactant weight ratios of the Labrafil M1944CS/Kolliphor HS15/Transcutol P combination. It should be noted that the non-ionic surfactant, Kolliphor HS15, used for the development of SNEDDS exhibits increased hydrophilicity (HLB = 15) necessary for the formation of o/w emulsions.
Maritsa Margaroni, Evgenia Tsanaktsidou, Maria Agallou, Costas Kiparissides, Olga Kammona, Evdokia Karagouni,
Development of a novel squalene/a-tocopherol-based self-emulsified nanoemulsion incorporating Leishmania peptides for induction of antigen-specific immune responses, International Journal of Pharmaceutics, 2023, 123621, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123621.
See also the interesting video on Vitamin E TPGS and read more here:

