Interaction of thermal and rheological parameters critical for the processability of polyvinyl alcohol-based systems during hot melt extrusion
Purpose
Hot melt extrusion (HME) is a pivotal pharmaceutical manufacturing process employed to enhance the bioavailability of poorly soluble active pharmaceutical ingredients (APIs) in oral dosage forms. Often the applicability of the process is limited by the melt viscosity. The upper limit is considered at about 10,000 Pas.3 Especially for high melting polymers it is of particular interest to explore concepts that can widen the process range.
Objectives
Identify suitable plasticizers in combination with polyvinyl alcohol (PVA) grades and investigate their influence on the melt viscosity. The aim is to assess correlations between thermal characteristics and key rheological parameters.
Methods
Materials
Polyvinyl alcohol (PVA, Parteck® MXP 3-82 and 4-88), glycerol (GLY), triacetin (TRI), triethylcitrate (TEC), polyethylene glycol (PEG 300), mannitol (MAN, Parteck® M200) and sorbitol (SOR, Parteck® Si150), from Millipore Sigma (Darmstadt Germany), plasticizer content at 10 wt%.
Melt rheology
Haake™ Mars™ Rheo 60 (Thermofisher Scientific, USA). Plate diameter = 25 mm, gap = 1 mm, sample mass ≈550 mg, viscosity and phase angle delta, were determined from 170 °C to 250 °C for PVA 4-88 blends, and from 150 °C to 230 °C for the PVA 3-82 blends, 2K/min, ɣ0 = 0.1%, f = 6.28 rad/s, n = 3.
Differential scanning calorimetry (DSC)
DSC 3+ (Mettler Toledo, USA) sample mass ≈5 mg, n=3, 1st heating cycle -80 °C to 210 °C, cooling cycle 210 °C to -80 °C, 2nd heating cycle -80 °C to 250 °C 10 K/min, 50 mL/min N2, 40 μl Al crucible, data evaluated with STARe Excellence Software.
Results
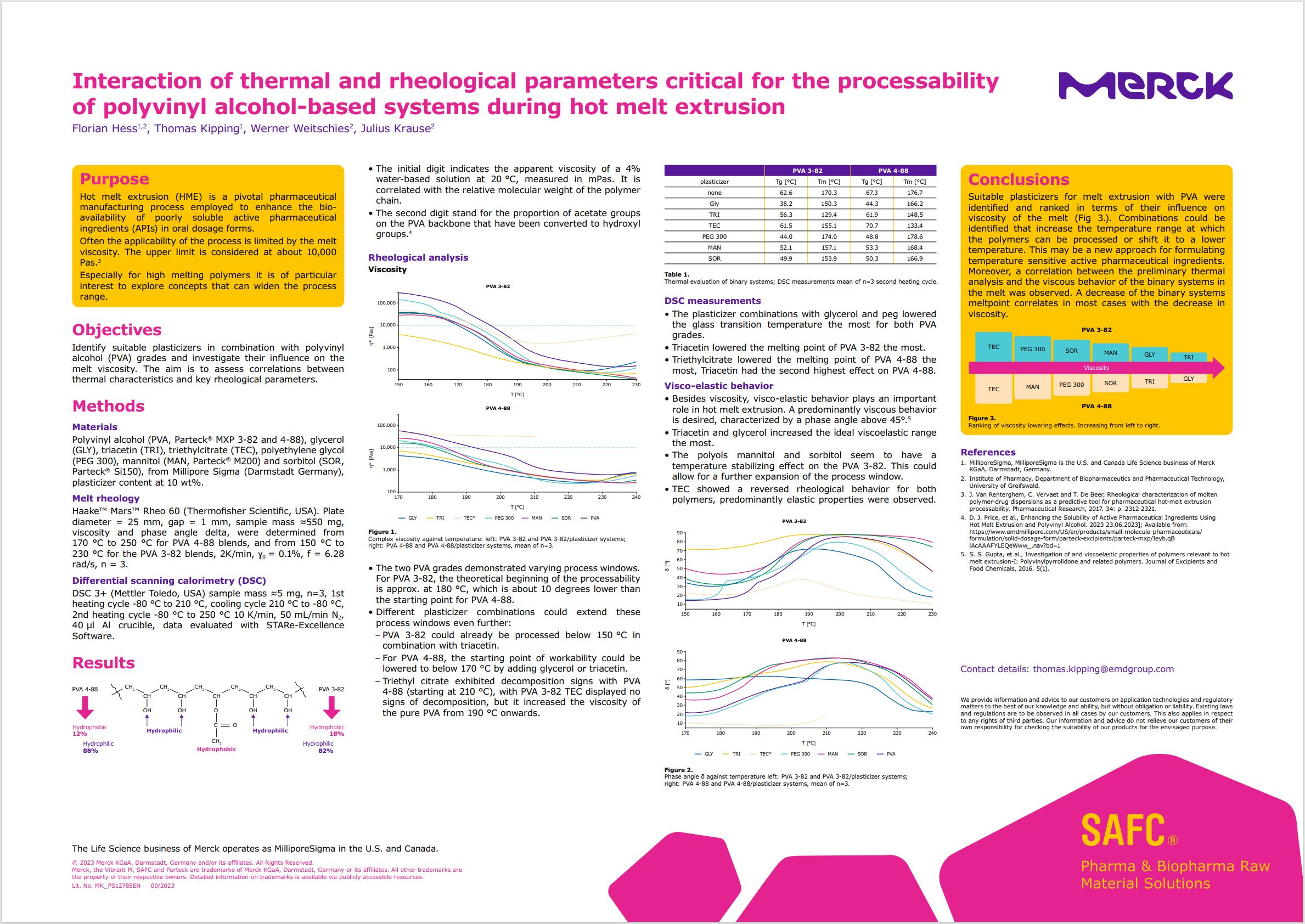
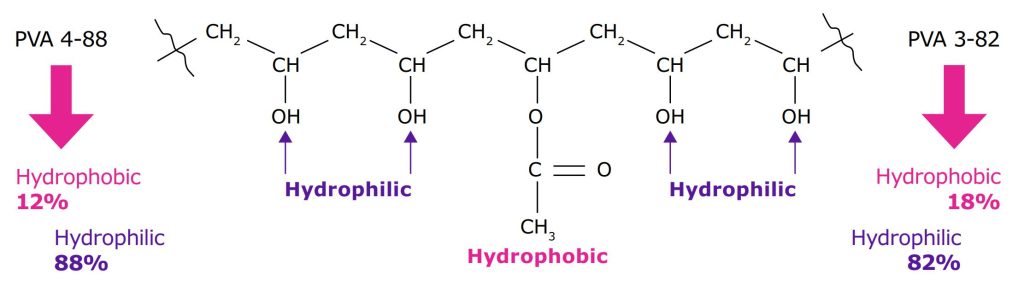
- The initial digit indicates the apparent viscosity of a 4% water-based solution at 20 °C, measured in mPas. It is correlated with the relative molecular weight of the polymer chain.
- The second digit stand for the proportion of acetate groups on the PVA backbone that have been converted to hydroxyl groups.4
Rheological analysis
Viscosity
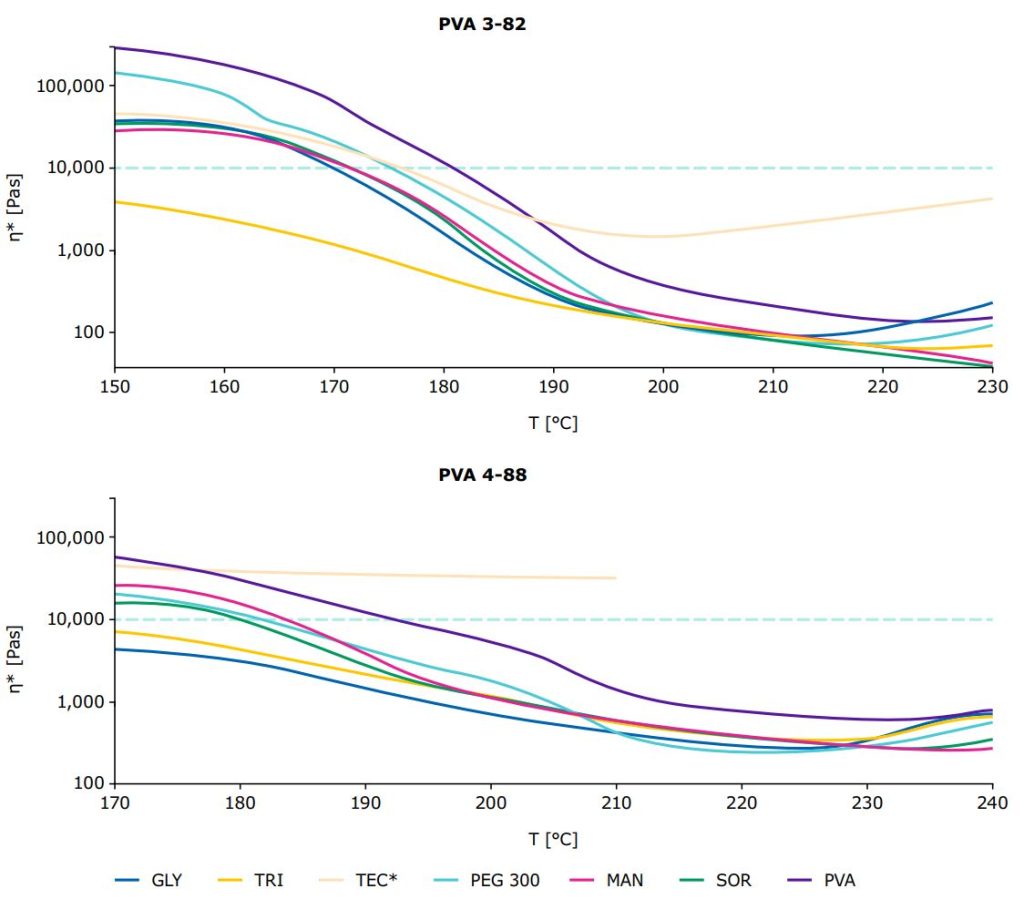
right: PVA 4-88 and PVA 4-88/plasticizer systems, mean of n=3.
- The two PVA grades demonstrated varying process windows. For PVA 3-82, the theoretical beginning of the processability is approx. at 180 °C, which is about 10 degrees lower than the starting point for PVA 4-88.
- Different plasticizer combinations could extend these process windows even further:
- PVA 3-82 could already be processed below 150 °C in combination with triacetin.
- For PVA 4-88, the starting point of workability could be lowered to below 170 °C by adding glycerol or triacetin.
- Triethyl citrate exhibited decomposition signs with PVA 4-88 (starting at 210 °C), with PVA 3-82 TEC displayed no signs of decomposition, but it increased the viscosity of the pure PVA from 190 °C onwards.
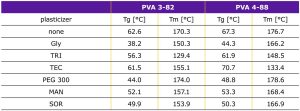
DSC measurements
- The plasticizer combinations with glycerol and peg lowrede the glass transition temperature the most for both PVA grades.
- Triacetin lowered the melting point of PVA 3-82 the most.
- Triethylcitrate lowered the melting point of PVA 4-88 the most, Triacetin had the second highest effect on PVA 4-88.
Visco-elastic behavior
- Besides viscosity, visco-elastic behavior plays an important role in hot melt extrusion. A predominantly viscous behavior is desired, characterized by a phase angle above 45°.5
- Triacetin and glycerol increased the ideal viscoelastic range the most.
- The polyols mannitol and sorbitol seem to have a temperature stabilizing effect on the PVA 3-82. This could allow for a further expansion of the process window.
- TEC showed a reversed rheological behavior for both polymers, predominantly elastic properties were observed
See the full brochure on “Interaction of thermal and rheological parameters” here
(click the picture to download the brochure)
Source: Merck brochure “Interaction of thermal and rheological parameters”
Do you need more information or a sample of excipients by Merck?


