Solid-in-oil nanodispersion as a novel topical transdermal delivery to enhance stability and skin permeation and retention of hydrophilic drugs l-ascorbic acid
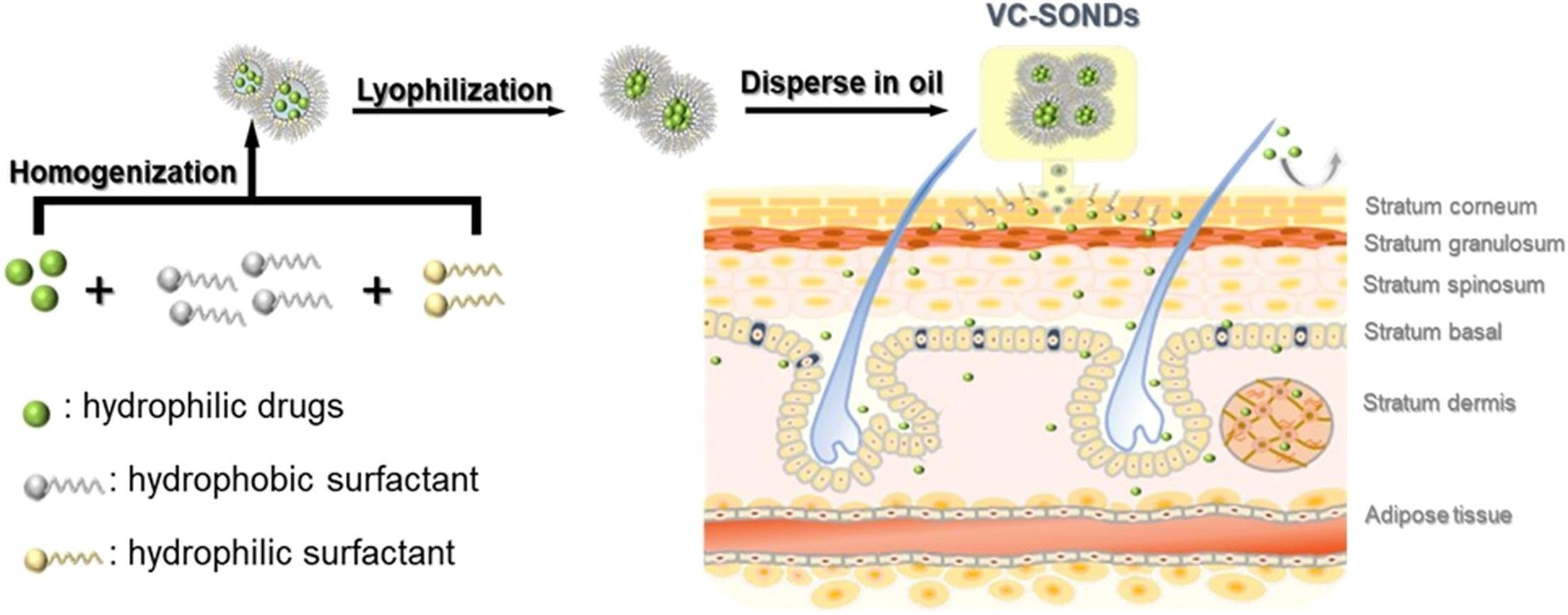
l-ascorbic acid (Vitamin C, VC)is the most abundant antioxidant in human skin. But its poor penetration into the skin and unstability limit the application. The aim of the study was to promote the topical skin permeation and retention of VC, increase the stability as well as effectiveness by a novel solid in oil nanodispersion. In the nanodispersions system, nano-sized particles of hydrophilic molecules are dispersed in an oil vehicle with the assistance of hydrophobic surfactants. The optimized formula composed of O170 and S1570 (12.5:1, w/w) showed high EE% of 98% and good stability. FTIR analysis confirmed that there may be hydrogen bond between VC and surfactants. The results of DSC, and XRD revealed that the drug was successfully encapsulated in the surfactants, which maintained the stability of drug.
By analyzing and fitting the release data in vitro, the drug release mechanism of SONDs was predicted as a multi-dynamic model. Skin permeation of VC was improved 3.43-fold for SONDs compared with VC aqueous solution, highlighting that the lipophilicity and nano size of the carrier more easily penetrated into the skin. Finally, the photoaging study revealed that topical application of VC-SONDs provided the highest skin protection compared UV and VC aqueous solution treated group which was evident by the normal thick epidermal morphology, no obvious melanocytes and the densely arranged dermal elastic fibers. These results demonstrated that the solid-in-oil nanodispersions may be a potential transdermal delivery system for hydrophilic bioactive ingredients.
Read more
Materials
l-ascorbic acid(VC) was obtained from Tokyo Chemical Industry Co., ltd. (Shanghai, China). Sucrose erucate (commercial name, ER290, HLB = 2), sucrose oleate (commercial name, O170, HLB = 1), sucrose laurate (commercial name, L195, HLB = 1), sucrose monolaureate (commercial name, L1695, HLB = 16) and sucrose stearate (commercial name, S1570, HLB = 15) were kindly provided by Mitsubishi-Kagaku Foods Co., ltd. (Tokyo, Japan). Isopropyl myristate (IPM) and squalene was procured from Aladdin
Yue Zhang, Wenxiu Pan, Dequan Wang, Han Wang, Yanting Hou, Meijuan Zou, Hongyu Piao, Solid-in-oil nanodispersion as a novel topical transdermal delivery to enhance stability and skin permeation and retention of hydrophilic drugs l-ascorbic acid, European Journal of Pharmaceutics and Biopharmaceutics, Volume 185, 2023, Pages 82-93, ISSN 0939-6411, https://doi.org/10.1016/j.ejpb.2023.02.004.

