Controlling drug release by introducing lipase inhibitor within a lipid formulation
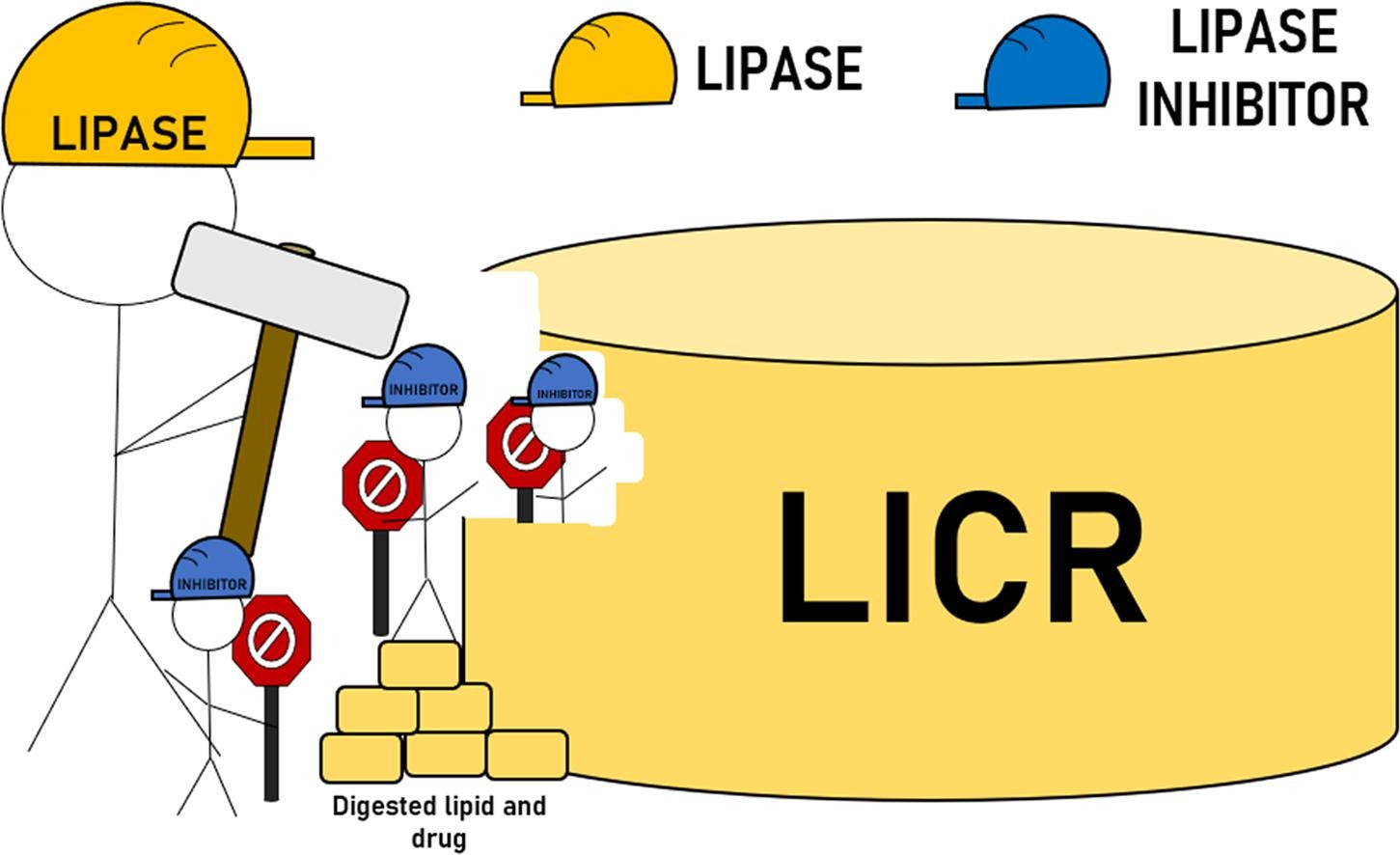
Drug overdose connected to marketed pharmaceutical products, particularly opioids, occurs at an alarming rate. Novel strategies through innovative formulation approaches that reduce the likelihood of overdose while allowing safe therapeutic outcomes are urgently required. The current study provides a proof-of-concept for a new formulation approach by co-formulating drug with a lipase inhibitor within a solid lipid formulation in order to prevent or reduce the harmful effects of taking multiple doses of an oral solid dose form. Lipase inhibitor controlled-release (LICR) formulations were created using a simple hot melt method to co-formulate the inhibitor (orlistat) and ibuprofen, as the model drug, within the lipid matrix. The digestion and drug release kinetics were determined using an in vitro lipolysis model. Above a threshold level of orlistat there was decreased digestibility of multiple doses of the LICR formulations, leading to reduced drug release. Upon administration of the formulations in capsules to rats, the LICR formulation displayed the lowest exposure of ibuprofen during the pharmacokinetic studies. This novel formulation approach shows promise in preventing accidental drug overdose after oral administration of multiple doses of formulation.
Download the research paper here: Controlling drug release by introducing lipase inhibitor within a lipid formulation
or continue here
Materials and Methods
Ibuprofen sodium salt (>98%), 4-bromophenylboronic acid (4-BPBA, >95%), Trizma® maleate (reagent grade), sodium taurodeoxycholate hydrate (NaTDC, ≥95%) and triethylamine (TEA, ≥99 %) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Gelucire® 43/01 (a mixture of C8-C18 di- and tri-acylglycerols and free polyethylene glycol esters) was obtained from Gattefossé (France). Hydrochloric acid (HCl, 36%) was purchased from LabServ (Longford, Ireland). Sodium chloride (>99%) was purchased from Chem Supply (South Australia, Australia). Orlistat was purchased from both Sigma-Aldrich (St. Louis, MO, USA) (>98%) and Selleck Chemicals (Texas, USA) (>97.6%). DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine, >99%) was purchased from Cayman Chemical (Michigan, USA). Calcium chloride dihydrate (>99%) and sodium hydroxide pellets (>97%) were obtained from Ajax Finechem (Seven Hills, NSW, Australia). Sodium azide (NaN3) (>99%), methanol, chloroform and acetonitrile (ACN) were obtained from Merck (Darmstadt, Germany). Orthophosphoric acid (OPA, >85%) was purchased from Univar (Illinois, USA). USP grade porcine pancreatin extract was purchased from Southern Biological (Nunawading, Victoria, Australia). Water used in this study was from a Milli-Q purification system (Billerica, USA).
Mubtasim Murshed, Anna Pham, Kapilkumar Vithani, Malinda Salim, Ben J. Boyd,
Controlling drug release by introducing lipase inhibitor within a lipid formulation,
International Journal of Pharmaceutics, Volume 623, 2022, 121958, ISSN 0378-5173,
https://doi.org/10.1016/j.ijpharm.2022.121958.

