Liposomal Encapsulation of Amoxicillin via Microfluidics with Subsequent Investigation of the Significance of PEGylated Therapeutics
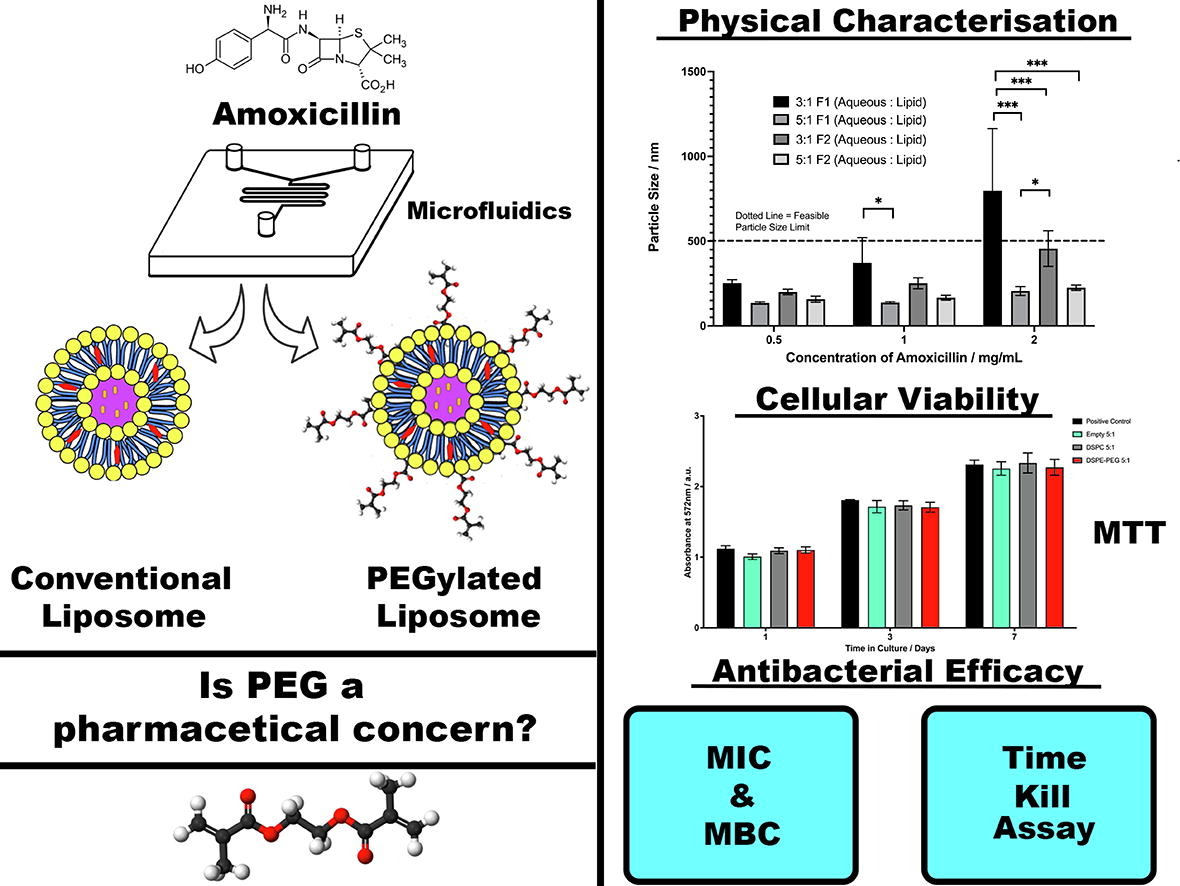
With an increasing concern of global antimicrobial resistance, the efforts to improve the formulation of a narrowing library of therapeutic antibiotics must be confronted. The liposomal encapsulation of antibiotics using a novel and sustainable microfluidic method has been employed in this study to address this pressing issue, via a targeted, lower-dose medical approach.The study focusses upon microfluidic parameter optimisation, formulation stability, cytotoxicity, and future applications. Particle sizes of circa. 130 nm, with viable short-term (28-day) physical stability were obtained, using two different non-cytotoxic liposomal formulations, both of which displayed suitable antibacterial efficacy. The microfluidic method allowed for high encapsulation efficiencies (≈77 %) and the subsequent in vitro release profile suggested high limits of antibiotic dissociation from the nanovessels, achieving 90% release within 72 hours.
Highlights
- Microfluidic formulation of amoxicillin produces homogenous, size-controlled liposomes.
- Particles produced are non-cytotoxic and allow for viable cell attachment.
- Liposomes display a high level of antimicrobial affect against both gram positive and negative bacteria.
- PEGylation of nanoparticles is assessed and determined to be beneficial for the formulations produced.
- Issues surrounding antimicrobial resistance could be circumvented using targeted therapy.
In addition to the experimental data, the growing use of poly(ethylene) glycol (PEG) within lipid-based formulations is discussed in relation to anti-PEG antibodies, highlighting the key pharmacological differences between PEGylated and non-PEGylated formulations and their respective advantages and drawbacks. It’s surmised that in the case of the formulations used in this study, the addition of PEG upon the liposomal membrane would still be a beneficial feature to possess owing to beneficial features such as stability, AB efficacy and the capacity to further modify the liposomal membrane.
Download the full article as PDF here Liposomal Encapsulation of Amoxicillin via Microfluidics with Subsequent Investigation of the Significance of PEGylated Therapeutics
or read it here
Materials
Cholesterol (Chol), and AMOX were purchased from Tokyo Chemical Industry (Tokyo, Japan). 1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC), phosphate buffered saline (PBS) tablets, ethanol (<99%), sodium phosphate monobasic (NaH2PO4), and acetic acid was purchased from Sigma-Aldrich (Poole, UK). N-(carbonyl-methoxypolyethylene glycol-2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine, sodium salt (DSPE-PEG2K) was obtained from Lipoid (Ludwigshafen Germany). McCoy’s 5A culture media with L-glutamine and Gentamicin were purchased from Lonza (Basel, Switzerland). Fetal bovine serum (FBS), heat inactivated South American origin and Trypsin EDTA were obtained from Gibco (Montana, US). Crystal violet and Sodium dodecyl sulfate was purchased from ThermoFisher (Massachusetts, US). Methanol and N,N-dimethylformamide (DMF) were purchased from ROMIL Ltd. (Cambridge ,UK). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was purchased from Invitrogen (Massachusetts, US). U-2 OS cell line, Staphylococcus aureus (S. aureus, ATCC 29213) and Proteus mirabilis (P. mirabilis, ATCC 51286) were purchased from ATCC (Virginia, US).
Edward Weaver, Robyn A. Macartney, Robyn Irwin, Shahid Uddin, Andrew Hooker, George A. Burke, Matthew P. Wylie, Dimitrios A. Lamprou, Liposomal Encapsulation of Amoxicillin via Microfluidics with Subsequent Investigation of the Significance of PEGylated Therapeutics, International Journal of Pharmaceutics, 2023, 123710, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123710.
Read more on “Amoxicillin” here:
- Rectal Bioavailability of Amoxicillin from Hollow-Type Suppositories
- Rectal bioavailability of amoxicillin sodium in rabbits
- Fabrication of floating capsule-in- 3D-printed devices as gastro-retentive delivery systems of amoxicillin