Lyophilization for Formulation Optimization of Drug-Loaded Thermoresponsive Polyelectrolyte Complex Nanogels from Functionalized Hyaluronic Acid
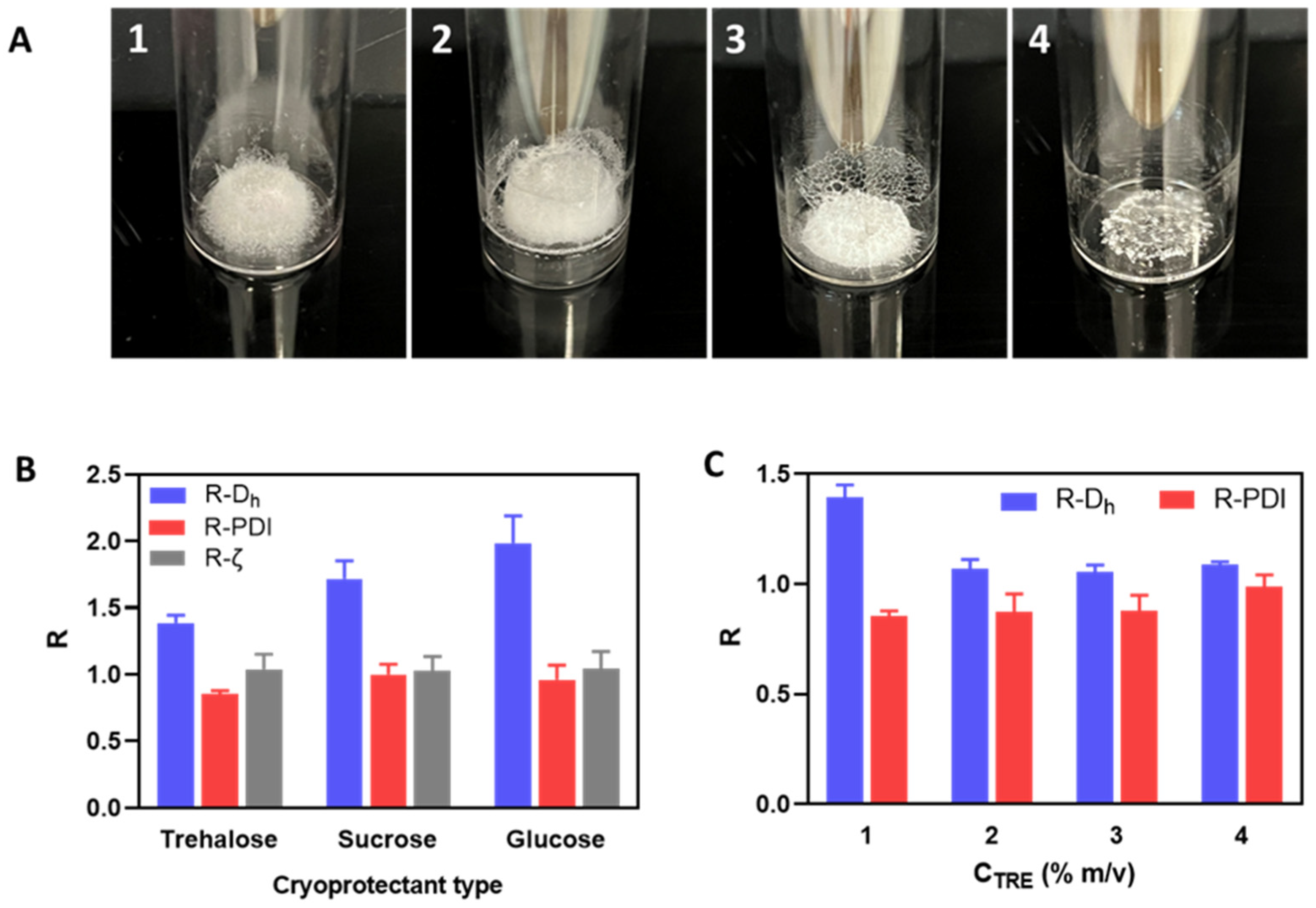
The lyophilization of nanogels is practical not only for their long-term conservation but also for adjusting their concentration and dispersant type during reconstitution for different applications. However, lyophilization strategies must be adapted to each kind of nanoformulation in order to minimize aggregation after reconstitution. In this work, the effects of formulation aspects (i.e., charge ratio, polymer concentration, thermoresponsive grafts, polycation type, cryoprotectant type, and concentration) on particle integrity after lyophilization and reconstitution for different types of polyelectrolyte complex nanogels (PEC-NGs) from hyaluronic acid (HA) were investigated. The main objective was to find the best approach for freeze-drying thermoresponsive PEC-NGs from Jeffamine-M-2005-functionalized HA, which has recently been developed as a potential platform for drug delivery.
It was found that freeze-drying PEC-NG suspensions prepared at a relatively low polymer concentration of 0.2 g.L−1 with 0.2% (m/v) trehalose as a cryoprotectant allow the homogeneous redispersion of PEC-NGs when concentrated at 1 g.L−1 upon reconstitution in PBS without important aggregation (i.e., average particle size remaining under 350 nm), which could be applied to concentrate curcumin (CUR)-loaded PEC-NGs for optimizing CUR content. The thermoresponsive release of CUR from such concentrated PEC-NGs was also reverified, which showed a minor effect of freeze-drying on the drug release profile.
Download the full article as PDF here Lyophilization for Formulation Optimization of Drug-Loaded Thermoresponsive Polyelectrolyte Complex Nanogels from Functionalized Hyaluronic Acid
or read it here
Materials
HA with a weight-average molecular weight (Mw) of 270,000 g.mol−1 was kindly offered by Givaudan (Pomacle, France). HA-M2005 obtained by grafting Jeffamine M-2005 (Huntsman, Houston, TX, USA) on HA with a degree of substitution of around 3.6% was retrieved from our previous work [4]. DEAE-D hydrochloride (Mw of 700,000 g.mol−1), α-PLL hydrobromide (Mw of 490,000 g.mol−1), CUR, phosphate-buffered saline (PBS) tablets, Tween 20, trehalose dihydrate, sucrose, glucose, and PEGs having number-average molecular weights (Mn) of 2000; 10,000 and 20,000 g.mol−1 were purchased from Sigma-Aldrich (Saint-Quentin-Fallavier, France). Ascorbic acid was purchased from Acros Organics (Schwert, Seitingen-Oberflacht, Germany). Water used for all experiments was purified by the Milli-Q system (Millipore, Burlington, MA, USA).
Le, H.V.; Dulong, V.; Picton, L.; Le Cerf, D. Lyophilization for Formulation Optimization of Drug-Loaded Thermoresponsive Polyelectrolyte Complex Nanogels from Functionalized Hyaluronic Acid. Pharmaceutics 2023, 15, 929. https://doi.org/10.3390/pharmaceutics15030929

