Machine vision-based non-destructive dissolution prediction of meloxicam-containing tablets
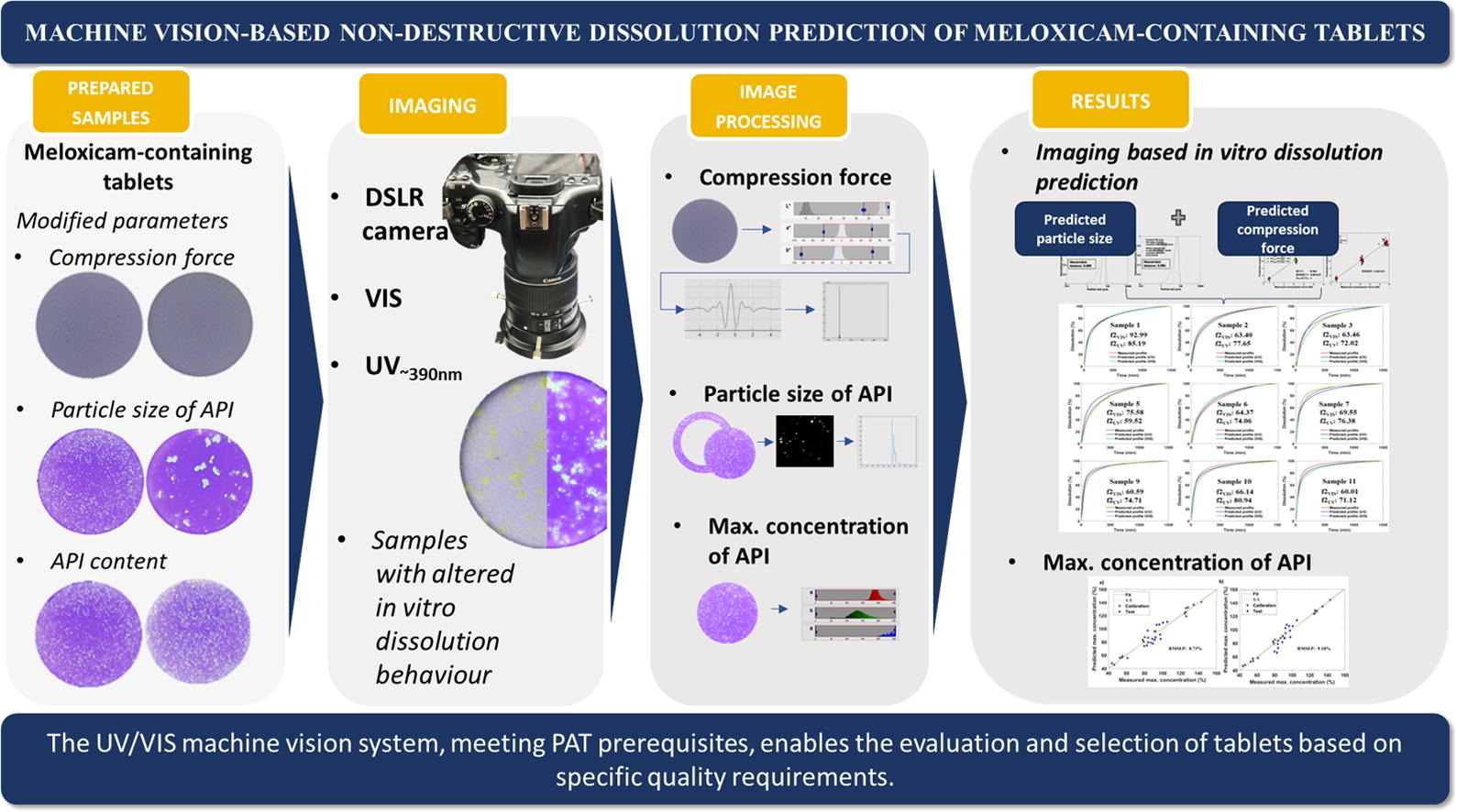
Machine vision systems have emerged for quality assessment of solid dosage forms in the pharmaceutical industry. These can offer a versatile tool for continuous manufacturing while supporting the framework of process analytical technology, quality-by-design, and real-time release testing. The aim of this work is to develop a digital UV/VIS imaging-based system for predicting the in vitro dissolution of meloxicam-containing tablets.
Highlights
- Innovative approach in dissolution prediction utilizing UV and VIS images.
- VIS images enable a non-destructive prediction of compression force.
- Particle size evaluation utilizing UV and VIS images for dissolution prediction.
- Utilizing machine vision to gather information on API content.
- Machine vision-based comprehensive quality assessment of tablets.
The alteration of the dissolution profiles of the samples required different levels of the critical process parameters, including compression force, particle size and content of the API. These process parameters were predicted non-destructively by multivariate analysis of UV/VIS images taken from the tablets. The dissolution profile prediction was also executed using solely the image data and applying artificial neural networks. The prediction error (RMSE) of the dissolution profile points was less than 5%.
The alteration of the API content directly affected the maximum concentrations observed at the end of the dissolution tests. This parameter was predicted with a relative error of less than 10% by PLS models that are based on the color components of UV and VIS images. In conclusion, this paper presents a modern, non-destructive PAT solution for real-time testing of the dissolution of tablets.
Download the full article as PDF here: Machine vision-based non-destructive dissolution prediction of meloxicam-containing tablets
or read more here
Materials
Meloxicam (MLX) was utilized as the active pharmaceutical ingredient (API), belonging to class 2 due to its high permeability and limited solubility in the Biopharmaceutics Classification System (BCS) (Simionato et al., 2018). Acetone, sodium hydroxide, and sodium-dihydrogen-phosphate were obtained from Sigma Aldrich (St. Louis, MO, USA). Vivapur 200 microcrystalline cellulose (MCC) was purchased from JRS Pharma (Rosenberg, Germany).
Preparation of Tablets
After weighing the components, powder mixtures were manually homogenized for 10 min. A total of 172 tablets were manually produced using a Dott Bonapace CPR 6 (Dott Bonapace, Italy) eccentric tablet press, within the compression force range of 5–15 kN, and with the API content ranging from 2.3 to 3.5 w/w% as stated in Table 1. The tablet press was equipped with a single concave punch.
Lilla Alexandra Mészáros, Lajos Madarász, Szabina Kádár, Máté Ficzere, Attila Farkas, Zsombor Kristóf Nagy, Machine vision-based non-destructive dissolution prediction of meloxicam-containing tablets, International Journal of Pharmaceutics, 2024, 124013, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.124013.
Read also our introduction article on Microcrystalline Cellulose here:


