A Novel Combined Dry Powder Inhaler Comprising Nanosized Ketoprofen-Embedded Mannitol-Coated Microparticles for Pulmonary Inflammations: Development, In Vitro–In Silico Characterization, and Cell Line Evaluation
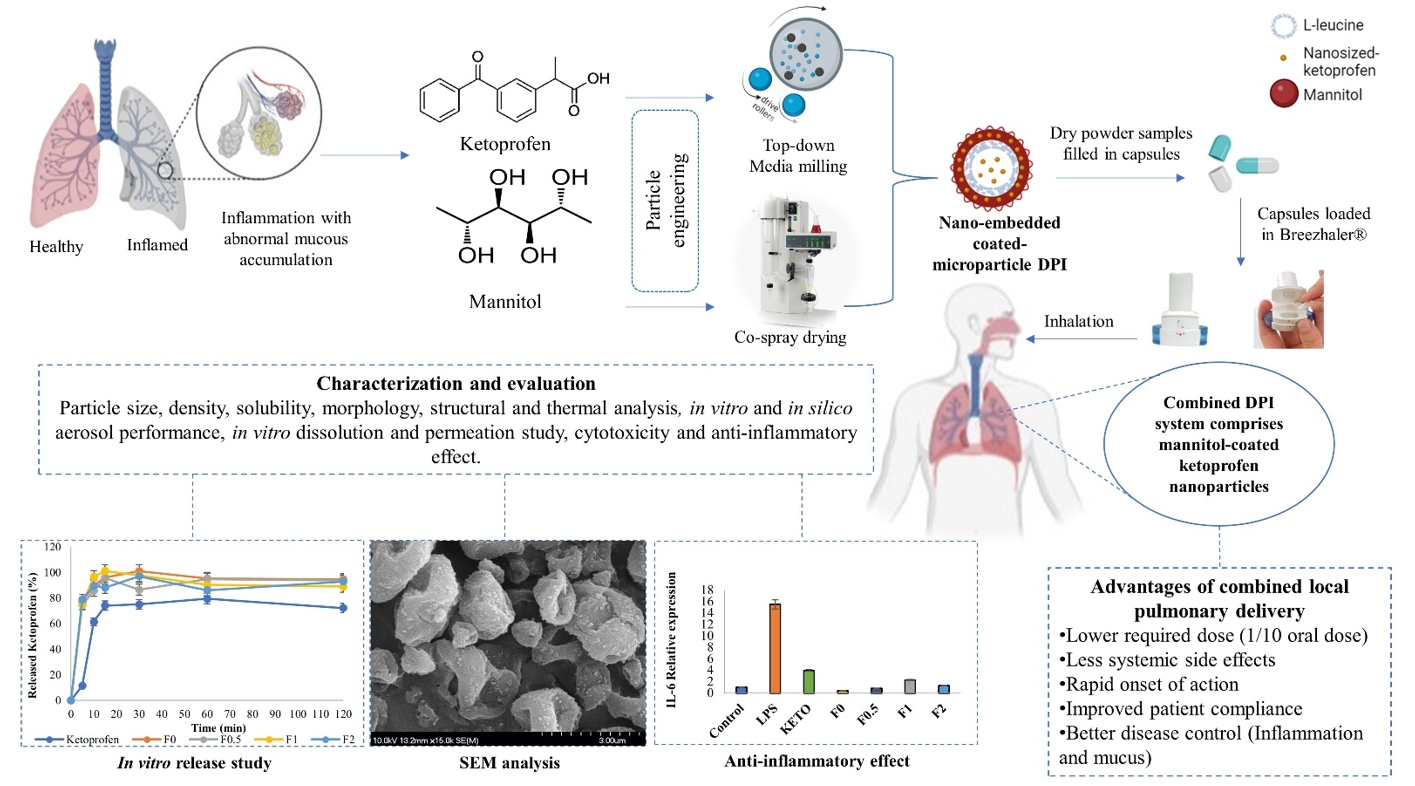
Pulmonary inflammations such as chronic obstructive pulmonary disease and cystic fibrosis are widespread and can be fatal, especially when they are characterized by abnormal mucus accumulation. Inhaled corticosteroids are commonly used for lung inflammations despite their considerable side effects. By utilizing particle engineering techniques, a combined dry powder inhaler (DPI) comprising nanosized ketoprofen-embedded mannitol-coated microparticles was developed.
A nanoembedded microparticle system means a novel advance in pulmonary delivery by enhancing local pulmonary deposition while avoiding clearance mechanisms. Ketoprofen, a poorly water-soluble anti-inflammatory drug, was dispersed in the stabilizer solution and then homogenized by ultraturrax. Following this, a ketoprofen-containing nanosuspension was produced by wet-media milling. Furthermore, co-spray drying was conducted with L-leucine (dispersity enhancer) and mannitol (coating and mucuactive agent). Particle size, morphology, dissolution, permeation, viscosity, in vitro and in silico deposition, cytotoxicity, and anti-inflammatory effect were investigated. The particle size of the ketoprofen-containing nanosuspension was ~230 nm. SEM images of the spray-dried powder displayed wrinkled, coated, and nearly spherical particles with a final size of ~2 µm (nano-in-micro), which is optimal for pulmonary delivery.
The mannitol-containing samples decreased the viscosity of 10% mucin solution. The results of the mass median aerodynamic diameter (2.4–4.5 µm), fine particle fraction (56–71%), permeation (five-fold enhancement), and dissolution (80% release in 5 min) confirmed that the system is ideal for local inhalation. All samples showed a significant anti-inflammatory effect and decreased IL-6 on the LPS-treated U937 cell line with low cytotoxicity. Hence, developing an innovative combined DPI comprising ketoprofen and mannitol by employing a nano-in-micro approach is a potential treatment for lung inflammations.
4.1. Materials
KETO (TCI chemicals, Shanghai, China) was used as a drug model. Poly-vinyl-alcohol PVA (ISP Customer Service GmBH, Cologne, Germany) was used as a stabilizer. SDS (VWR chemicals, Leuven, Belgium) was employed as a surfactant. In spray-drying process, MAN (Molar Chemicals Ltd., Budapest, Hungary) was applied as a coating material, while LEU (AppliChem GmbH, Darmstadt, Germany) was exploited as a dispersity enhancer. Distilled water used in this study was obtained from Milli-Q, Millipore, Merck KGaA, Darmstadt, Germany.
Download the full study as PDF here: A Novel Combined Dry Powder Inhaler Comprising Nanosized Ketoprofen-Embedded Mannitol-Coated Microparticles for Pulmonary Inflammations: Development, In Vitro–In Silico Characterization, and Cell Line Evaluation
or read it here
Banat, H.; Csóka, I.; Paróczai, D.; Burian, K.; Farkas, Á.; Ambrus, R. A Novel Combined Dry Powder Inhaler Comprising Nanosized Ketoprofen-Embedded Mannitol-Coated Microparticles for Pulmonary Inflammations: Development, In Vitro–In Silico Characterization, and Cell Line Evaluation. Pharmaceuticals 2024, 17, 75.
https://doi.org/10.3390/ph17010075

