MeltSerts Technology (Brinzolamide ocular inserts via hot-melt extrusion): QbD-steered development, molecular dynamics, in vitro, ex vivo and in vivo studies
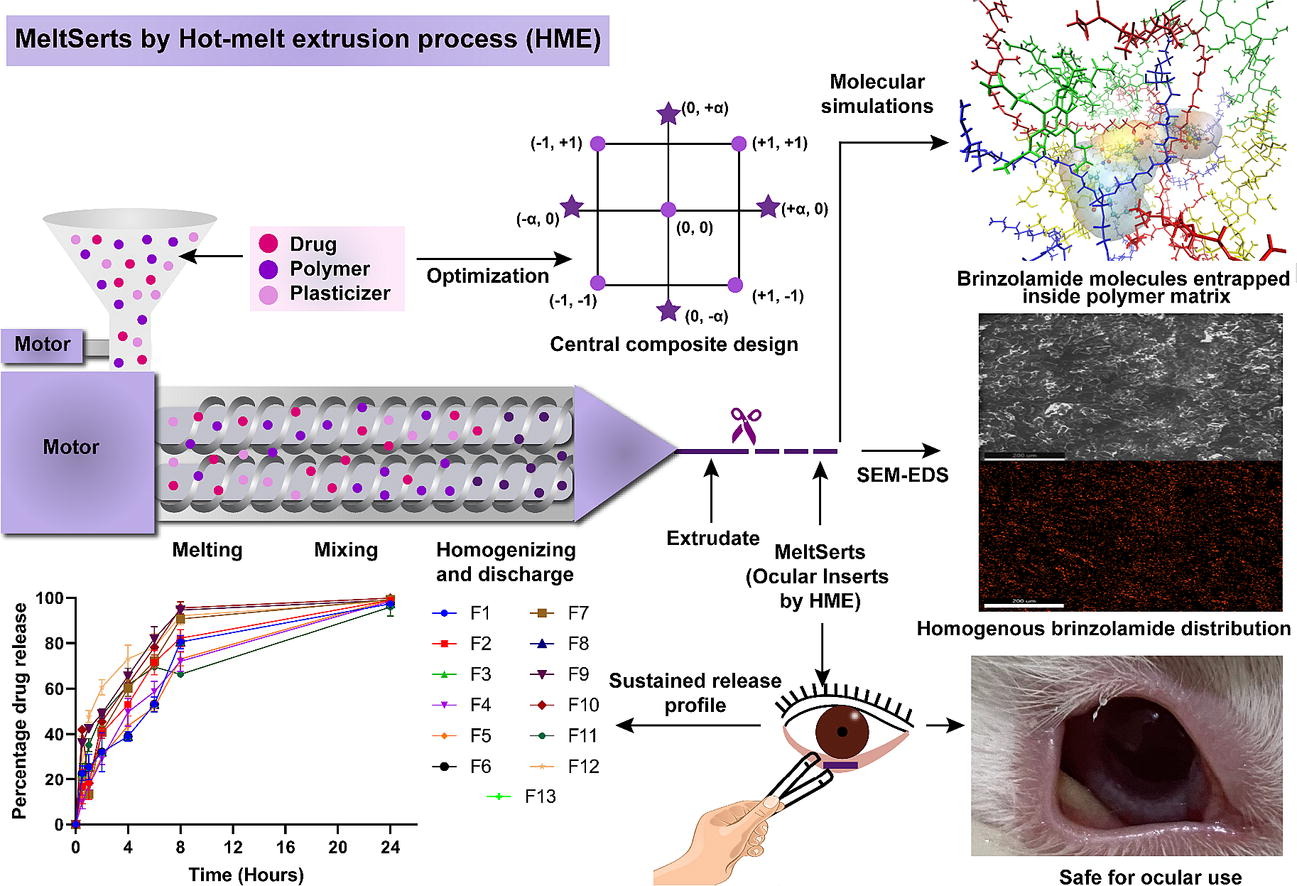
The research work aimed to develop a robust sustained release biocompatible brinzolamide (BRZ)-loaded ocular inserts (MeltSerts) using hot-melt extrusion technology with enhanced solubility for glaucoma management. A 32 rotatable central composite design was employed for the optimization of the MeltSerts to achieve sustained release. The effect of two independent factors was examined: Metolose® SR 90SH-100000SR (HPMC, hydroxypropyl methyl cellulose) and Kolliphor® 407 (Poloxamer 407, P407). The drug release (DR) of BRZ at 0.5h and 8h were adopted as dependent responses. The factorial analysis resulted in an optimum composition of 50.00% w/w of HPMC and 15.00% w/w of P407 which gave % DR of 9.11 at 0.5h and 69.10 at 8h.
Furthermore, molecular dynamic simulations were performed to elucidate various interactions between BRZ, and other formulation components and it was observed that BRZ showed maximum interactions with HPC and HPMC with an occupancy of 92.82 and 52.87%, respectively. Additionally, molecular docking studies were performed to understand the interactions between BRZ and mucoadhesive polymers with ocular mucin (MUC-1). The results indicated a docking score of only -5.368 for BRZ alone, whereas a significantly higher docking score was observed for the optimized Meltserts -6.977, suggesting enhanced retention time of the optimized MeltSerts. SEM images displayed irregular surfaces, while EDS analysis validated uniform BRZ distribution in the optimized formulation.
The results of the ocular irritancy studies both ex vivo and in vivo demonstrated that MeltSerts are safe for ocular use. The results indicate that the developed MeltSerts Technology has the potential to manufacture ocular inserts with cost-effectiveness, one-step processability, and enhanced product quality. Nonetheless, it also offers a once-daily regimen, consequently decreasing the dosing frequency, preservative exposure, and ultimately better glaucoma management.
Read more here
Materials
BRZ was provided as a gift sample by Cipla India Pvt. Ltd. Kolliphor® 407 (Poloxamer 407, P407), Kollidon® 30 LP (polyvinyl pyrrolidone, PVP), and Kolliphor® EL (Polyoxyl-35 castor oil, KLP) were kindly provided by BASF India Pvt. Ltd. KlucelTM HF and Metolose® SR 90SH-100000SR (100000 mPas) were kindly donated by Ashland India Pvt. Ltd., and ShinEtsu Pvt. Ltd. (Tokyo, Japan), respectively. DMSO was purchased from Rankem Chemicals.
Srushti Tambe, Divya Jain, Ravi Rawat, Suraj Mali, Mario Angelo Pagano, Anna Maria Brunati, Purnima Amin,
MeltSerts Technology (Brinzolamide ocular inserts via hot-melt extrusion): QbD-steered development, molecular dynamics, in vitro, ex vivo and in vivo studies, International Journal of Pharmaceutics, 2023, 123579, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123579.
Get more informatinon on Quality by Design (QbD) here:


