Impact of dry coating lactose as a brittle excipient on multi-component blend processability
Abstract
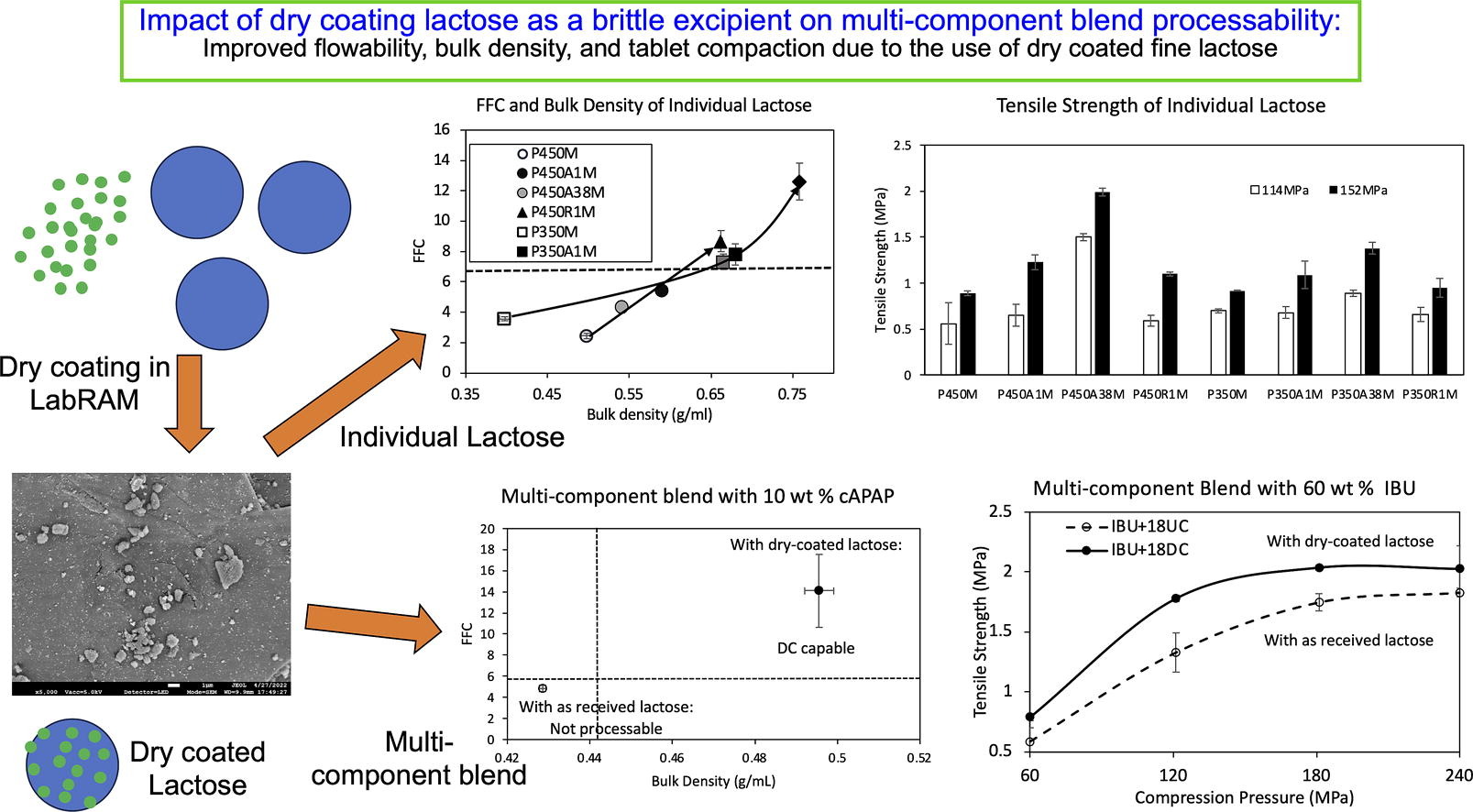
Previous work demonstrated the benefits of dry coating fine-grade microcrystalline cellulose (MCC) for enabling direct compression (DC), a favored tablet manufacturing method, due to enhanced flowability while retaining good compactability of placebo and binary blends of cohesive APIs. Here, fine brittle excipients, Pharmatose 450 (P450, 19 μm) and Pharmatose 350 (P350, 29 μm), having both poor flowability and compactability are dry coated with silica A200 or R972P to assess DC capability of multi-component cohesive API (coarse acetaminophen, 22 μm, and ibuprofen50, 47 μm) blends. Dry coated P450 and P350 not only attained excellent flowability and high bulk density but also heightened tensile strength hence processability, which contrasts with reported reduction for dry coated ductile MCC. Although hydrophobic R972P imparted better flowability, hydrophilic A200 better enhanced tensile strength, hence selected for dry coating P450 in multi-component blends that included fine Avicel PH-105. For coarse acetaminophen blends, substantial bulk density and flowability increase without any detrimental effect on tensile strength were observed; a lesser amount of dry coated P450 was better. Increased flowability, bulk density, and tensile strength, hence enhanced processability by reaching DC capability, were observed for 60 wt% ibuprofen50, using only 18 wt% of the dry coated P450, i.e. 0.18 wt% silica in the blend.
Highlights
- Lactose, a brittle excipient, dry coated with R972P/A200 for property enhancements
- Coated fine lactose had better flow & tensile strength, in contrast to ductile MCC
- Hydrophobic silica better for flow, hydrophilic better for tensile strength (TS)
- Cohesive API + dry coated lactose blends had excellent flow without compromising TS
- Less dry coated lactose better, 0.18 wt% silica from 18 wt% lactose better than more.
Introduction
Tablets have long been preferred as a solid dosage form for drug delivery due to their stability, cost-effectiveness, ease of administration, and excellent patient compliance (Awad et al., 2022, Sam and Fokkens, 1997, Sheth et al., 1980). Among various tablet manufacturing methods, direct blending and direct compression (DB-DC) route is preferred owing to its fewer processing steps, easier validation, and improved drug stability (Bolhuis and Anthony Armstrong, 2006). However, adoption of DB-DC option requires the APIs as well as blend to have good bulk density, flowability, and tabletability (Han et al., 2011, Huang et al., 2015a, Huang et al., 2015b, Huang et al., 2017, Jallo et al., 2012, Jivraj et al., 2000, Shi et al., 2011). Unfortunately, not all active pharmaceutical ingredients (APIs) exhibit suitable tableting characteristics on their own for direct compression (G Mirani et al., 2011, Sastry et al., 2000), necessitating the use of excipients to facilitate DB-DC tableting. That has led to added emphasis on the use of higher functionality excipients that enable enhanced formulations (Garg et al., 2013, Gohel and Jogani, 2005, Rojas et al., 2012, Sastry et al., 2000), motivating the development of novel excipients (Gupta et al., 2006).
Co-processed excipients have emerged as a solution to improve tabletability and flowability for direct compression (Garg et al., 2013, Garg et al., 2015, Gohel and Jogani, 2005, Rojas et al., 2012). These powders are composed of two or more parent particulate materials physically assembled at the sub-particle level to modify their characteristics (Gohel and Jogani, 2005). However, existing manufacturing processes for co-processed excipients may present concerns such as the environmental burden due to the need for using solvents and liquids, additional milling or drying steps, low yield rates, or high silica concentration (Carlin, 2008, Maury et al., 2005, Ståhl et al., 2002). Excessive silica content, particularly exceeding 2 wt% in the blend, is not FDA compliant (FDA, 2015) and can have detrimental effects on tablet properties (Chattoraj et al., 2011, Chen et al., 2018b, FDA, 2015, Van Veen et al., 2005, Zhou et al., 2012). Another likely limitation of co-processed excipient mixtures is their fixed ratio of constituents, which may not be optimal for specific APIs and desired tablet doses for the most effective formulation development (Bolhuis and Chowhan, 1996, Gohel and Jogani, 2005).
Dry coating, which has proven to be an efficient and robust method for improving the required properties of excipients or APIs, such as bulk density, flowability, and tabletability (Chattoraj et al., 2011, Chen et al., 2018a, Chen et al., 2018b, Chen et al., 2020, Chen et al., 2019, Han et al., 2011), may offer an alternative to above-described co-processed excipients. Dry coating involves using a suitable dry high-intensity mixing process to apply mechanical force to coat small guest particles onto the surface of larger host particles (Chen et al., 2008). Numerous studies have reported dry coating of ductile excipients, for example, microcrystalline cellulose (MCC), and demonstrated the success of dry-coated MCC formulations for placebo (Chattoraj et al., 2011, Chen et al., 2018a, Chen et al., 2018b) as well as binary blends (Chen et al., 2018b). Previous reports also demonstrated significant improvements in the flowability of individual MCC powders and further demonstrated improved flowability, bulk density, and tabletability of placebo and binary blends (Chen et al., 2018a, Chen et al., 2018b, Chen et al., 2020, Chen et al., 2019). However, the topic of dry coating effectiveness in multi-component blends or on the blends containing dry coated brittle excipients such as lactose has not been reported.
This research aims to fill this gap by examining the dry coating effect on lactose, one of the most widely used brittle pharmaceutical excipients due to its excellent filler properties and its ability to expedite liquid penetration followed by dissolution that promotes tablet disintegration and dissolution (Gomes et al., 2021, Hebbink and Dickhoff, 2019, Maclean et al., 2021). There are examples of previous work concerning dry coating of lactose by itself, where the flowability and bulk density of individual lactose was found to be improved due to dry coating (Kunnath et al., 2021, Kunnath et al., 2023). However, unlike previous work involving dry coating of MCC as an excipient (Chattoraj et al., 2011, Chen et al., 2018b, Chen et al., 2020), there are no reports concerning the effect of dry coating for lactose as an excipient and the impact of dry coating on its tabletability. Unlike ductile MCC, lactose is a brittle material (Roberts, 2011). It would be clearly important to understand if lactose being brittle, behaves differently from MCC where dry coating resulted in reduced tensile strength (TS), attributed to the lower surface energy of the hydrophilic silica coating as compared to the surface energy of MCC (Chen et al., 2018a, Chen et al., 2018b, Chen et al., 2019, Etzler et al., 2011). In fact, the reported work with MCC avoided using even lower surface energy hydrophobic silica R972P due to the tabletability loss concerns. An interesting recent paper has reported that while dry coating of MCC resulted in a loss of the placebo tablet TS, the blends including dry coated MCC did not have such adverse effects (Chen et al., 2019). Such investigation for the effect of dry coated lactose in blends has not been explored and hence it is considered in the current paper. Further, generally speaking, MCC enhances tabletability through ductile deformation and high bonding strength, while brittle lactose achieves it through fragmentation and a high bonding area (Sun, 2011). The combination of plastic deformation of MCC and lactose fragmentation contributes to their synergistic compatibility (Al-Ibraheemi et al., 2013) hence study of their combination where lactose is dry coated would also be a worthwhile consideration in the current paper, which has motivated examination of multi-component blends.
Consequently, this study investigates the effect of dry coating on lactose particles with various silica types and their amounts on lactose by itself in its multi-component blends containing APIs. As a part of such investigation, the impact of lactose particle size and relative loading in blend formulations on bulk density, flowability, and tabletability are explored. To be consistent with the previous papers where dry coated MCC exhibited good improvement in flowability and bulk density, while keeping low TS loss (Chen et al., 2018a, Chen et al., 2018b), this paper considers lactose of the same size as MCC in previous papers, i.e., 20 μm and 30 μm. Selecting two sizes for lactose allows examination of the influence of silica amount, type, and lactose particle size, on individual lactose flowability and bulk density, as well as tabletability of their placebos. Pharmatose 450, which is the finer of the two lactose types, is selected here for its higher TS although it poses challenges due to its finer size with respect to flowability and bulk density. Coarse Acetaminophen (CAPAP) and Ibuprofen 50 (IBU) will serve as the model APIs in the blend formulation; the rationale behind their selection is presented in the results section. By addressing such objectives, this research aims to contribute valuable insights into the application of dry-coated lactose as a novel co-processed excipient along with uncoated MCC in multi-component tablet formulations.
Read more here
Materials
Pharmatose 450 (P450) and Pharmatose 350 (P350), both as received, were generously provided by DFE Pharma Corporation, USA. In the process of dry coating, hydrophilic Aerosil A200 silica (A200) and hydrophobic Aerosil R972P (R972P) silica were utilized, also donated by Evonik Corporation, USA. Magnesium Stearate (MgSt), a lubricant, was obtained from Mallinckrodt Inc., USA. The model drugs selected for this study were Coarse Acetaminophen (CAPAP) and Ibuprofen 50 (IBU), acquired from Changshu.
To produce tablets of multi-component blends containing IBU, a compaction simulator (STYL’One, MEDELPHARM, Beynost, France) was employed. The “DEFAULT CYCLE – 2 Compressions” cycle was utilized, with the pre-compression step.
Zhixing Lin, Bian Cabello, Rajesh N. Davé, Impact of dry coating lactose as a brittle excipient on multi-component blend processability, International Journal of Pharmaceutics, 2024, 123921, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.123921.
Read also our introduction article on Lubricants here:


