Nanoparticle-Based Adjuvants and Delivery Systems for Modern Vaccines
Ever since the development of the first vaccine, vaccination has had the great impact on global health, leading to the decrease in the burden of numerous infectious diseases. However, there is a constant need to improve existing vaccines and develop new vaccination strategies and vaccine platforms that induce a broader immune response compared to traditional vaccines. Modern vaccines tend to rely on certain nanotechnology platforms but are still expected to be readily available and easy for large-scale manufacturing and to induce a durable immune response.
(click to enlarge)
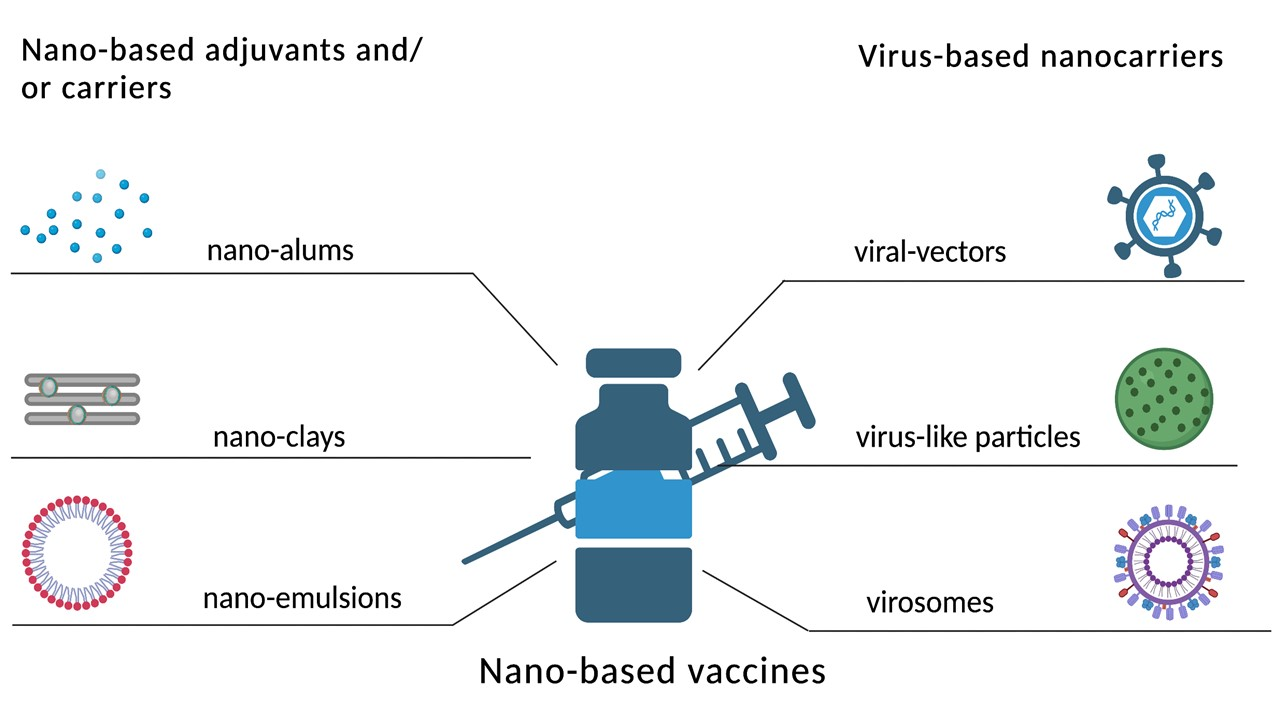
In this review, we present an overview of the most promising nanoadjuvants and nanoparticulate delivery systems and discuss their benefits from technological and immunological standpoints as well as their objective drawbacks and possible side effects. The presented nano alums, silica and clay nanoparticles, nanoemulsions, adenoviral-vectored systems, adeno-associated viral vectors, vesicular stomatitis viral vectors, lentiviral vectors, virus-like particles (including bacteriophage-based ones) and virosomes indicate that vaccine developers can now choose different adjuvants and/or delivery systems as per the requirement, specific to combatting different infectious diseases.
Download the full article as PDF here Nanoparticle-Based Adjuvants and Delivery Systems for Modern Vaccines
or read it here
Excipients mentioned in the paper besides others: mannitol, phospholipids, lipid nanoparticles, polysorbates
Filipić, B.; Pantelić, I.; Nikolić, I.; Majhen, D.; Stojić-Vukanić, Z.; Savić, S.; Krajišnik, D. Nanoparticle-Based Adjuvants and Delivery Systems for Modern Vaccines. Vaccines 2023, 11, 1172. https://doi.org/10.3390/vaccines11071172
Watch our webinar “Quality Requirements for Phospholipid Excipients in Liposomal Formulations” here:


