Structure and Fate of Nanoparticles Designed for the Nasal Delivery of Poorly Soluble Drugs
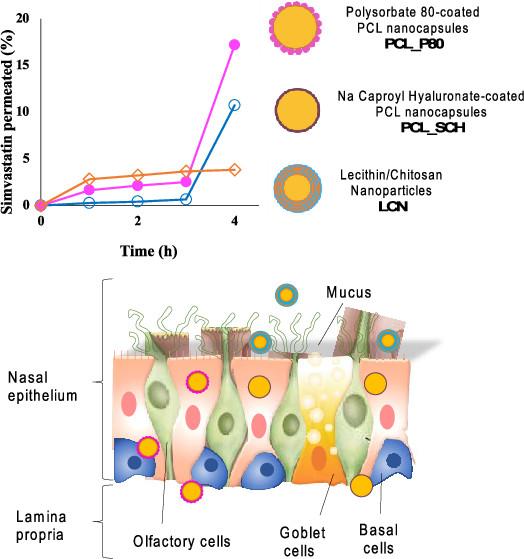
Nanoparticles are promising mediators to enable nasal systemic and brain delivery of active compounds. However, the possibility of reaching therapeutically relevant levels of exogenous molecules in the body is strongly reliant on the ability of the nanoparticles to overcome biological barriers. In this work, three paradigmatic nanoformulations vehiculating the poorly soluble model drug simvastatin were addressed: (i) hybrid lecithin/chitosan nanoparticles (LCNs), (ii) polymeric poly-ε-caprolactone nanocapsules stabilized with the nonionic surfactant polysorbate 80 (PCL_P80), and (iii) polymeric poly-ε-caprolactone nanocapsules stabilized with a polysaccharide-based surfactant, i.e., sodium caproyl hyaluronate (PCL_SCH).
The three nanosystems were investigated for their physicochemical and structural properties and for their impact on the biopharmaceutical aspects critical for nasal and nose-to-brain delivery: biocompatibility, drug release, mucoadhesion, and permeation across the nasal mucosa. All three nanoformulations were highly reproducible, with small particle size (∼200 nm), narrow size distribution (polydispersity index (PI) < 0.2), and high drug encapsulation efficiency (>97%). Nanoparticle composition, surface charge, and internal structure (multilayered, core–shell or raspberry-like, as assessed by small-angle neutron scattering, SANS) were demonstrated to have an impact on both the drug-release profile and, strikingly, its behavior at the biological interface. The interaction with the mucus layer and the kinetics and extent of transport of the drug across the excised animal nasal epithelium were modulated by nanoparticle structure and surface.
In fact, all of the produced nanoparticles improved simvastatin transport across the epithelial barrier of the nasal cavity as compared to a traditional formulation. Interestingly, however, the permeation enhancement was achieved via two distinct pathways: (a) enhanced mucoadhesion for hybrid LCN accompanied by fast mucosal permeation of the model drug, or (b) mucopenetration and an improved uptake and potential transport of whole PCL_P80 and PCL_SCH nanocapsules with delayed boost of permeation across the nasal mucosa. The correlation between nanoparticle structure and its biopharmaceutical properties appears to be a pivotal point for the development of novel platforms suitable for systemic and brain delivery of pharmaceutical compounds via intranasal administration.
Download the full article as a PDF here or read it here
Materials: Chitosan (Chitoclear FG, 95% deacetylation degree, 105 mPa·s viscosity, and ∼150,000 g/mol MW) was supplied by Primex (Siglufjordur, Iceland) and used without further purifications. Soybean lecithin (Lipoid S45) was obtained from Lipoid AG (Ludwigshafen, Germany). Pharmaceutical-grade oils Labrafac Lipophile WL 1349 (medium-chain triglycerides, EP) and Maisine 35-1 (glycerol monolinoleate) were a kind gift from Gattefossé (Saint-Priest, France). Poly-ε-caprolactone (PCL, MW 14 kDa) was supplied by Fluka-Sigma-Aldrich (St. Louis, MO, USA). Sodium caproyl hyaluronate (MW 200 kDa) was obtained from Contipro Biotech S.r.o. (Dolní Dobrouč, Czech Republic). The surfactants polysorbate 80 (Tween 80) and sorbitan monostearate 60 (Span 60) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Pharmaceutical-grade caprylic/capric triglyceride oil (Miglyol 812) was supplied by Caesar & Loretz GmbH (Mainz, Germany). Simvastatin (MW 418.6 g/mol) was provided by Polichimica (Bologna, Italy). Bovine serum albumin (BSA), mucin from porcine stomach type III (partially purified powder), deuterium oxide (D2O, 99.9 atom % D), and dialysis tubing cellulose acetate (14,000 Da molecular weight cutoff, MWCO) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The human nasal septum carcinoma cell line RPMI 2650 (batch CCL-30) was purchased from American Type Culture Collection (ATCC) (Manassas, VA, USA). Minimal essential medium (MEM), fetal bovine serum (FBS), and nonessential amino acid solution were provided by Life Technologies (ThermoFisher Scientific, Waltham, MA, USA). All Transwell cell culture inserts and other consumables were purchased from Corning Inc. Life Science (Corning, NY, USA). Ultrapure water (Purelab Flex, ELGA-Veolia LabWater, Italy) was used in all experiments, except for the specified cases where D2O was used. All other chemical reagents were of analytical grade.
Article information: Adryana Rocha Clementino, Giulia Pellegrini, Sabrina Banella, Gaia Colombo, Laura Cantù, Fabio Sonvico, and Elena Del Favero. Molecular Pharmaceutics, Article ASAP. DOI: 10.1021/acs.molpharmaceut.1c00366

