In Vitro and In Vivo Evaluation of Composite Oral Fast Disintegrating Film: An Innovative Strategy for the Codelivery of Ranitidine HCl and Flurbiprofen
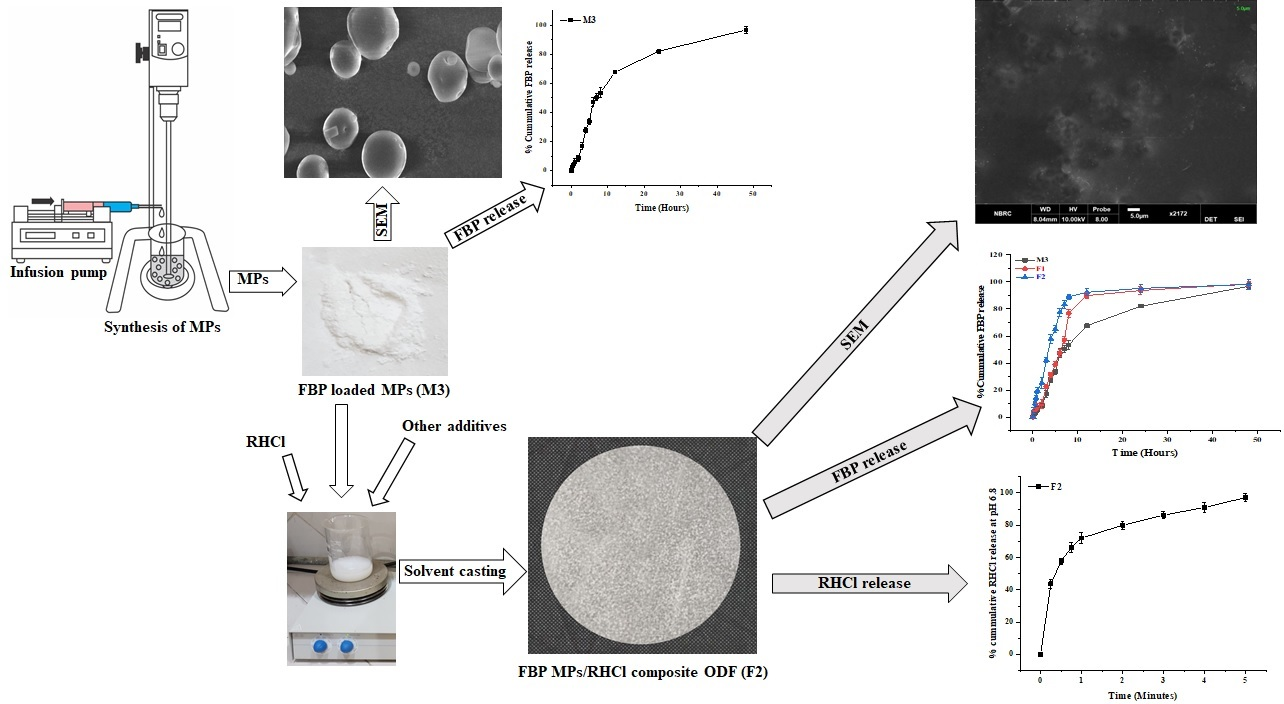
Here, we evaluate the feasibility of co-loading plain ranitidine hydrochloride (RHCl) and microencapsulated flurbiprofen (FBP) in a Lycoat® RS780-based oral fast disintegrating film (ODF). These films were developed by the solvent casting method to minimize the adverse effects of FBP and reduce the dosage form burden on patients. Optimized FBP microparticles (M3) with an average size of 21.2 ± 9.2 µm were loaded alone (F1) and in combination with plain RHCl (F2) in the composite ODF. All films were evaluated physicomechanically and physicochemically. These films were resilient, flexible, and disintegrated within thirty seconds. SEM images showed intact FBP microparticles in both formulations and, moreover, did not observe an interaction between the drug and film components.
Microencapsulated FBP was released in a controlled manner over 48 h from the proposed formulations, while RHCl was released within 5 min from F2. After in vitro evaluation, formulations were also tested for in vivo anti-inflammatory activity, cytokine (TNF-α and IL-6) levels, and gastroprotective effects in rats. The anti-inflammatory activity and gastroprotective effect of F2 were markedly higher than pure FBP and other synthesized formulations (M3 and F1). The average score of gastric lesions was in the order of pure FBP (15.5 ± 1.32) > M3 (8 ± 2) > F1 (1 ± 0.5) > F2 (0.5 ± 0) > control (0). Additionally, F2 showed a sustained anti-inflammatory effect up to 10 h in the rat paw edema model.
Furthermore, F2 also markedly reduced TNF-α and IL-6 levels. Conclusively, the Lycoat® RS780-based composite film could be a promising carrier for the co-loading of microencapsulated FBP with RHCl. In the future, an optimized formulation (F2) could be capable of countering the issues related to multiple drug administration in geriatric patients and evading the gastric irritation associated with FBP.
Download the full article here In Vitro and In Vivo Evaluation of Composite Oral Fast Disintegrating Film: An Innovative Strategy for the Codelivery of Ranitidine HCl and Flurbiprofen
or read more here
Materials
Flurbiprofen (FBP) and ranitidine HCl (RHCl) was obtained from Axis Pharmaceuticals (Pakistan). Lycoat® RS780 and Pearlitol Flash® (Mannitol Starch) were received as gift samples from Roquette Frères (France). Polyvinyl alcohol (PVA 1500), polyethylene glycol (PEG 400), propylene glycol (PG), and dichloromethane (DCM) were obtained from Daejung Chemicals and Metals Co., Ltd. (Siheung, Republic of Korea). Glycerin (GLY) was obtained from Sigma-Aldrich (Darmstadt, Germany). All other excipients and chemicals were of an analytical grade.
Rashid, A.; Khalid, S.H.; Irfan, M.; Asghar, S.; Rizg, W.Y.; Sabei, F.Y.; Alfayez, E.; Alkharobi, H.; Safhi, A.Y.; Hosny, K.M.; et al. In Vitro and In Vivo Evaluation of Composite Oral Fast Disintegrating Film: An Innovative Strategy for the Codelivery of Ranitidine HCl and Flurbiprofen. Pharmaceutics 2023, 15, 1987. https://doi.org/10.3390/pharmaceutics15071987
Read more on Orally Disintegrating Tablets (ODTs) here:


