Development of a New Jelly Coating Technology (Oral Jelly Coating) to Improve Prescribed Medication Adherence
Oral drug delivery is currently the gold standard in the pharmaceutical industry, regarded as the safest, most convenient and most economical method of drug delivery with the highest patient compliance [1, 2]. Among oral formulations, tablets and capsules are the most preferred dosage forms, but there are several limitations with these, including choking and swelling discomfort in geriatric and pediatric patients [3, 4]. From a pharmaceutical aspect, tablets are the most commonly prescribed dosage form as they offer a convenient form of drug administration based on the following: tablet uniformity, stability throughout extended and diverse storage conditions, and the ability to be easily produced using high-speed compression, labelling, and packaging technology. In reality, tablet production technology is constantly undergoing improvements to enhance the ability to deliver with precision, a desired drug in a dosage form intended for immediate or extended therapeutic effects [5, 6].
However, some groups of patients may find it difficult to swallow tablets or capsules. These groups include the elderly, children, and patients with neurodevelopmental disorders, as well as patients with nausea or who are on reduced liquid- or food-intake diets [7, 8]. Over the last several decades, from the perspective of formulation design, changes in tablet shape (downsizing and distinctive shaped tablets) and coatings (sugar coating) have been investigated. Recently, new formulation technologies such as orally disintegrating tablets (OD tablets), micro tablet-type granules, jelly dosage forms and film dosage forms have made it possible to create dosage forms that are easier to take [2, 9, 10]. The technological progress of OD tablets has been noteworthy, and many pharmaceutical manufacturers have established their own unique technology ranging from the original mold molding (first generation) to wet pressure molding (second generation) and dry pressure molding technology (third generation) [11, 12, 13].
Although advantages of OD tablets are significant, Abey FB and Urgurlu T have summarized several challenges and limitations of OD tablets, i.e., their relatively weak mechanical strength, difficulty in maintaining palatability by masking bitterness, protection from moisture during manufacturing and storage, and the necessity for measures that take into consideration absorption from the oral mucosa [14]. In fact, there is a clinical report on OD tablets showing that the tablet often disintegrated in the oral cavity and remained in the pharynx, resulting in incomplete absorption of the prescribed dose of the drug [15]. Technology has been developed to reduce the size of the medication into granules to make it easier to take, and to coat the surface with a gelling component to make it slippery in the oral cavity. Gelation induced mini-tablets; GEMTAB (mini-tablets film-coated with a gelling agent), have been developed and launched in recent years by a Japanese pharmaceutical company. Elsewhere, mini-tablets that swell just after coming into contact with water have been also investigated as another option to improve ingestion [16]. However, at present it is difficult to guarantee the content uniformity of individual mini-tablets because the tableting technology for precisely loading material into small multiple dies has not been well established [17]. Accordingly, mini-tablets are marketed as granular tablets, in which more than 20 tablets are enclosed in a “unit-dose package” [18, 19].
On the other hand, products to assist in swallowing, such as thickening agents or medication-aid jellies, have become frequently used in medical institutions and nursing facilities in recent years. It is reported that such products may contribute to reducing the bitterness of the drug [20] and may promote normal swallowing action [21]. The usefulness of thickening agents as swallowing aids has been studied, and it has been shown that when people with dysphagia use thickening agents, they may reduce the speed of swallowing in the pharynx and help prevent aspiration [22, 23]. However, it has been noted that thickening agents and medication-aid jellies may change the pharmacokinetics of active agents due to extending tablet disintegration time [24-26].
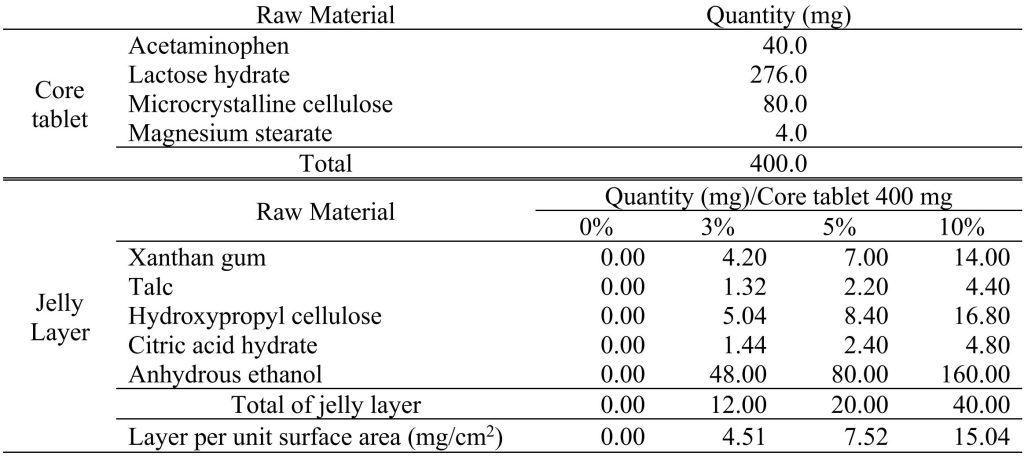
Table 1. Formulation of the core tablet and the jelly coating tablet for evaluating dissolution profile
Considering the above, we set out to develop a new tablet coating technology. We aimed to create a new coating technology that allows the tablet surface to change quickly into a jelly-like state after a patient takes the tablet, giving it the same slipperiness as if it were taken with thickening agents, thus allowing it to easily pass through the pharynx. The basic structure of our new oral jelly coating (hereinafter referred to as “OJ-coated tablet”) is threelayered, as shown in Fig. 1. The structure was designed to have a foaming layer containing carbonate as the innermost layer (1st layer) closest to the core tablet containing the drug, and a jelly layer containing the jelly material and acidic substances as the outermost layer (3rd layer). In addition, a separation layer (2nd layer) was set in between both layers to prevent direct contact between the acidic substances and carbonates during manufacturing and storage. With this design, it is expected that problems caused by OD tablets disintegrating in the oral cavity can be solved. As a result, there will be no change in the original disintegration properties of the tablet. It is also expected there will be no changes in pharmacokinetics. Our preliminary results show that the OJ coating technology can be applied to tablets of normal size and acceptable storage stability, being unaffected by humidity.
Download the full article as PDF here: Development of a New Jelly Coating Technology (Oral Jelly Coating) to Improve Prescribed Medication Adherence
Materials
Hypromellose (HPMC, TC-5E 3 mPa/s, TC-5M 4.5 mPa/s, manufactured by Shin-Etsu Chemical Co., Ltd.), sodium bicarbonate (SHC, sodium hydrogen carbonate, manufactured by AGC Inc.), xanthan gum (XG, San Ace S, manufactured by San-Ei Gen F.F.I. Inc.), hydroxypropyl cellulose (HPC-SL, made by Nippon Soda Co., Ltd.), citric acid hydrate (CAH, citric acid hydrate, made by Showa Kako Co., Ltd.) and talc (ML115, made by Fuji Talc Industries, Ltd.) were used as a coating material. In addition, anhydrous ethanol (anhydrous ethanol, Konishi Co., Ltd.) was used as the solvent for the coating preparation. Acetaminophen (N-acetyl-p-aminophenol: APAP, Tokyo Chemical Industry Co., Ltd.) was used as the test drug in the core tablet. In addition, lactose hydrate (Dilactose R, Freund Corporation), microcrystalline cellulose (Ceolus PH-102, Asahi Kasei Corporation) and magnesium stearate (Plant, Taihei Chemical Industry Co.) were used as a core tablet.
Source: Junya Yamashita, Shota Asai, Hiroki Shingaki, Masakane Hayakawa, Development of a New Jelly Coating Technology (Oral Jelly Coating) to Improve Prescribed Medication Adherence, Biological and Pharmaceutical Bulletin Advance Publication by J-STAGE, DOI:10.1248/bpb.b23-0062,
See also our overview article on ODT: