Enteric-coating film effect on the delayed drug release of pantoprazole gastro-resistant generic tablets
Abstract
Background: Enteric coating films in acidic labile tablets protect the drug molecule from the acidic environment of the stomach. However, variations in the excipients used in the coating formulation may affect their ability to provide adequate protection. This study is the first to investigate the potential effects of coating materials on the protective functionality of enteric coating films for pantoprazole (PNZ) generic tablets after their recall from the market.
Methods: A comparative analysis was conducted between generic and branded PNZ products, using pure drug powder for identification. The in vitro release of the drug was evaluated in different pH media. The study also utilized various analytical and thermal techniques, including differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier-transform infrared (FTIR), and confocal Raman microscopy.
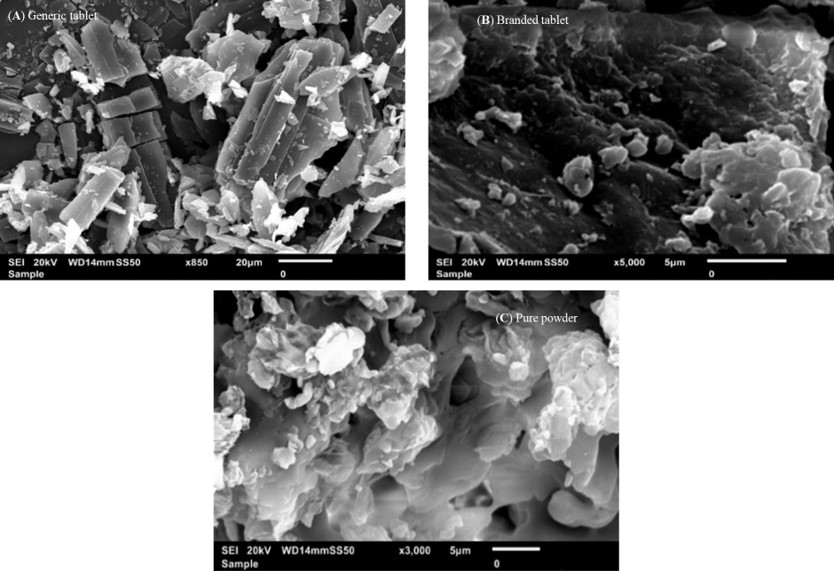
Results: The in vitro assessment results revealed significant variations in the release profile for the generic product in acidic media at 120 min. DSC and TGA thermal profile analyses showed slight variation between the two products. XRD analysis exhibited a noticeable difference in peak intensity for the generic sample, while SEM revealed smaller particle sizes in the generic product. The obtained spectra profile for the generic product displayed significant variation in peaks and band intensity, possibly due to impurities. These findings suggest that the excipients used in the enteric coating film of the generic product may have affected its protective functionality, leading to premature drug release in acidic media. Additionally, the presence of polysorbate 80 (P-80) in the brand product might improve the properties of the enteric coating film due to its multi-functionality.
Conclusions: In conclusion, the excipients used in the brand product demonstrated superior functionality in effectively protecting the drug molecule from acidic media through the enteric coating film, as compared to the generic version.
Introduction
Enteric coating films play a crucial role in pharmaceutical formulations as they are specifically designed to provide protection from premature releases of the drug molecule in acidic media.1,2 This protective function is particularly essential for drugs that are susceptible to degradation in acidic conditions, such as erythromycin,3 ampicillin,4 and penicillin G antibiotics,5 as well as certain proton pump inhibitors class of drugs, including omeprazole,4 Pantoprazole sodium sesquihydrate (PNZ),6 and esomeprazole.5,7 By forming a protective barrier, enteric coating films ensure the drugs reach their intended site of action intact.5,7 Moreover, enteric coating films also serve to prevent local irritation of the stomach mucosa caused by certain acidic drugs,8,9 including NSAIDs, like diclofenac10 and valproic acid.9 This feature is particularly important for enhancing patient tolerance and reducing potential side effects.8–10
Enteric-coated properties for tablets are commonly achieved through the use of various polymers and additives.11–13 Anionic polymers containing carboxyl groups are frequently employed to achieve the desired enteric effects.14 These polymers are insoluble under low pH conditions but exhibit solubility in intestinal fluid as the pH increases due to the ionization of acidic functional groups, resulting in polymer swelling.11,14 Common examples of anionic polymers used for enteric coating include cellulose acetate,15 polyvinyl acetate,16 hydroxypropyl methylcellulose,17,18 and methacrylic acid copolymers.11–13 Additives play a crucial role in polymer formulations, enhancing mechanical properties,19 modifying film permeability,19 facilitating film formation,12 and improving processing.12,20 Several commonly used additives serve these purposes effectively. For instance, plasticizers like Polysorbate 80 (P-80),21 tributyl citrate,22 and diethyl phthalate23 increase film flexibility, reduce brittleness, and influence drug release by minimizing crack formation.12,24 Anti-adherent materials, such as Talc25,26 and Glyceryl monostearate,27 are employed to reduce film tackiness and prevent substrate agglomeration.12,28 Additionally, surfactants like P-80,29 sorbitan monooleate,30 and sodium dodecyl sulfate31 are utilized to emulsify water-insoluble plasticizers, improve substrate wettability, and stabilize suspensions, thereby enhancing the overall properties of the enteric coat film.12,29
Generic drugs are manufactured by pharmaceutical companies in accordance with FDA.32,33 These regulations mandate that generic drugs have the same active ingredients, dosage form, strength, and route of administration as their brand-name counterparts and must be bioequivalent.26,32,34 However, certain variations in excipients are permitted.35,36 Although generic drugs are generally considered bioequivalent, some enteric-coated generic drugs have shown compromised functionality in protecting the drug molecule, resulting in premature drug release in simulated acidic media at physiological pH,37–39 such as omeprazole enteric-coated capsules,39 diclofenac enteric-coated tablets,38 lansoprazole enteric-coated tablets,40 and PNZ enteric-coated tablets.37
In this study, PNZ enteric-coated tablets were chosen as the model generic drug for comparison with the branded one, which was used as a reference. The generic product of PNZ was selected due to the reported issues that led to its recall from the local market. The chemical name of PNZ is Sodium 5-(difluoromethoxy)-2-(3,4-dimethoxy-2-pyridinyl)methyl)sulfincyl]-1H-benzimidazole sesquihydrate.41 It is primarily used as an anti-ulcer agent to treat duodenal and gastric ulcers.11,42 PNZ is classified as a class III drug according to the Biopharmaceutics Classification System (BCS), which indicates high solubility and low permeability.43,44 PNZ has a molecular weight of 432.4 g/mol11 and a melting point of 149-150°C.11,45 It is highly soluble in water,46 slightly soluble in chloroform,11 and practically insoluble in n-hexane.11 PNZ has a pKa value of 3.55 and a LogP value of 2.11.47 PNZ is a drug that is susceptible to degradation in the acidic environment of the stomach.48 therefore, commercially available enteric-coated tablets or capsules of PNZ are used to avoid the drug molecule degradation in acidic environments, ensuring its effectiveness.37,49
Several research studies have reported a reduction in the function of the enteric coating film, attributed to multiple possible factors.2,50–52 Therefore, maintaining the integrity of the enteric coating film primarily relies on the type and quantity of polymers and additives used for the film coating, as well as the manufacturing process.2,53 Several research studies have emphasized the importance of analyzing and evaluating the quality and functionality of enteric-coated medications using a range of analytical and thermal techniques.37,39,45 For instance sesquihydrate PNZ generic medication showed a different thermal profile than monohydrate PNZ when subjected to differential scanning calorimetry (DSC) and thermal gravimetric analysis (TGA).45 Fourier transforms infrared (FTIR) and Raman spectroscopy also revealed a significant difference in the spectra between monohydrate and sesquihydrate forms of PNZ drug molecule.45 In another study, premature drug release from enteric-coated generic PNZ tablets was observed in acidic media during in vitro dissolution evaluation.37 Furthermore, scanning electron microscopy (SEM) and X-ray diffraction (XRD) examinations demonstrated the absence of enteric coating on some granules of generic omeprazole capsules.39 These findings emphasize the importance of selecting suitable type and amount of ingredients to formulate the enteric coating film, as well as implementing an appropriate manufacturing process.
Therefore, the aim of this study was to find out the effect of coating film materials on the protection of PNZ drug molecules in acidic pH media. Both the brand and generic products of PNZ were utilized to compare any possible differences, with the brand product serving as the reference. Hence, the pure powder of PNZ was used for the drug identification process. To achieve these objectives, a range of analytical and thermal techniques, including DSC, TGA, XRD, SEM, FTIR, and Confocal Raman Microscopy, were used. Additionally, the in vitro drug release rate was evaluated in different pH media. To the best of our knowledge, no prior studies have been conducted on generic PNZ after has been recalled from the local market.
Download the full article as PDF here Enteric-coating film effect on the delayed drug release of pantoprazole gastro-resistant generic tablets
or read more here
Arafat M, Sakkal M, Bostanudin MF et al. Enteric-coating film effect on the delayed drug release of pantoprazole gastro-resistant generic tablets [version 1; peer review: awaiting peer review]. F1000Research 2023, 12:1325 (https://doi.org/10.12688/f1000research.140607.1)
See the webinar:
“Rational Selection of Cyclodextrins for the Solubilization of Poorly Soluble Oral Drugs”, 8. November 2023:
Get more information & register here for free:


