Parafilm® M and Strat-M® as skin simulants in in vitro permeation of dissolving microarray patches loaded with proteins
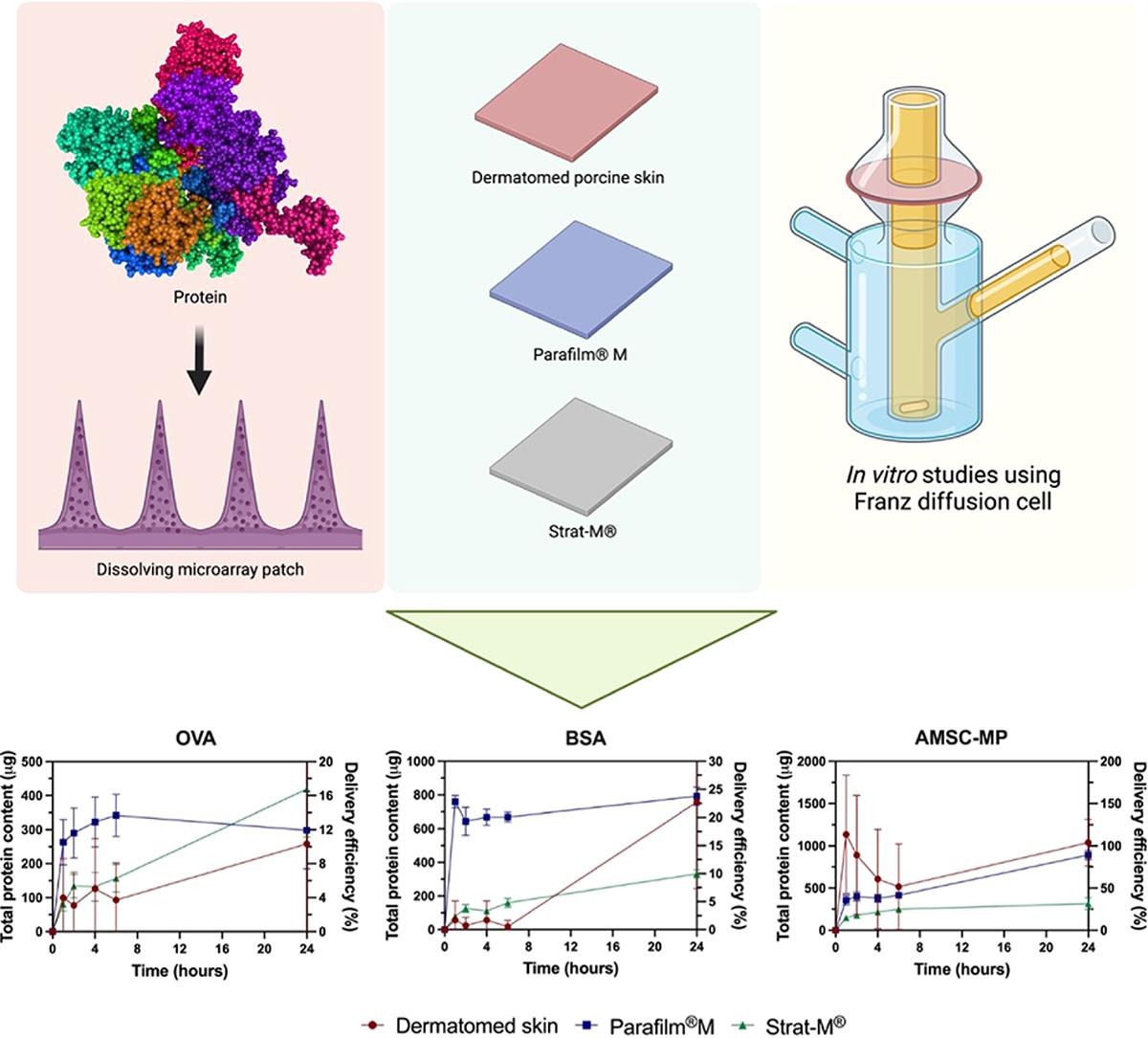
In vitro permeation studies play a crucial role in early formulation optimisation before extensive animal model investigations. Biological membranes are typically used in these studies to mimic human skin conditions accurately. However, when focusing on protein and peptide transdermal delivery, utilising biological membranes can complicate analysis and quantification processes.
This study aims to explore Parafilm®M and Strat-M® as alternatives to dermatomed porcine skin for evaluating protein delivery from dissolving microarray patch (MAP) platforms. Initially, various MAPs loaded with different model proteins (ovalbumin, bovine serum albumin and amniotic mesenchymal stem cell metabolite products) were prepared. These dissolving MAPs underwent evaluation for insertion properties and in vitro permeation profiles when combined with different membranes, dermatomed porcine skin, Parafilm®M, and Strat-M®. Insertion profiles indicated that both Parafilm®M and Strat-M® showed comparable insertion depths to dermatomed porcine skin (in range of 360–430 µm), suggesting promise as membrane substitutes for insertion studies.
In in vitro permeation studies, synthetic membranes such as Parafilm®M and Strat-M® demonstrated the ability to bypass protein-derived skin interference, providing more reliable results compared to dermatomed neonatal porcine skin. Consequently, these findings present valuable tools for preliminary screening across various MAP formulations, especially in the transdermal delivery of proteins and peptides.
Download the full article as PDF here Parafilm® M and Strat-M® as skin simulants in in vitro permeation of dissolving microarray patches loaded with proteins
or read it here
Materials
Albumin (chicken egg, ovalbumin) and bovine serum albumin (BSA) (lyophilised powder), poly(vinyl alcohol) (PVA) (MW 9–10 kDa, 80% hydrolysed) and poly(vinyl pyrrolidone) (PVP) K90 (MW 360 kDa) were purchased from Sigma-Aldrich (Dorset, UK). Amniotic mesenchymal stem cell metabolite products (AMSC-MP) lyophilised powder was obtained from the Stem Cell Research and Development Center, Airlangga University, Indonesia. Poly (vinyl pyrrolidone) (PVP) K29/32 (MW 58 kDa) were provided by Ashland (Kidderminster, UK). Strat-M® transdermal diffusion test model was purchased from Merck (Darmstadt, Germany). Parafilm® M was purchased from Amcor (Zürich, Switzerland). All other chemicals and materials were of analytical grade and purchased from Sigma-Aldrich (Dorset, UK) or Fisher Scientific (Loughborough, UK). Neonatal porcine skin was obtained from stillborn piglets less than 24 h after birth, rinsed in phosphate buffer saline (PBS pH 7.4), dermatomed to a thickness of 350 µm, and kept frozen at −20°C until use.
Qonita Kurnia Anjani, Avelia Devina Calista Nainggolan, Huanhuan Li, Andang Miatmoko, Eneko Larrañeta, Ryan F. Donnelly, Parafilm® M and Strat-M® as skin simulants in in vitro permeation of dissolving microarray patches loaded with proteins, International Journal of Pharmaceutics, Volume 655, 2024, 124071, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.124071.
See our next webinar:
“Rethinking the development of controlled release formulations and manufacturing processes”
Date: 30th of April, Time: 3:00 pm (Amsterdam, Berlin)


