Development of a PAT platform for the prediction of granule tableting properties
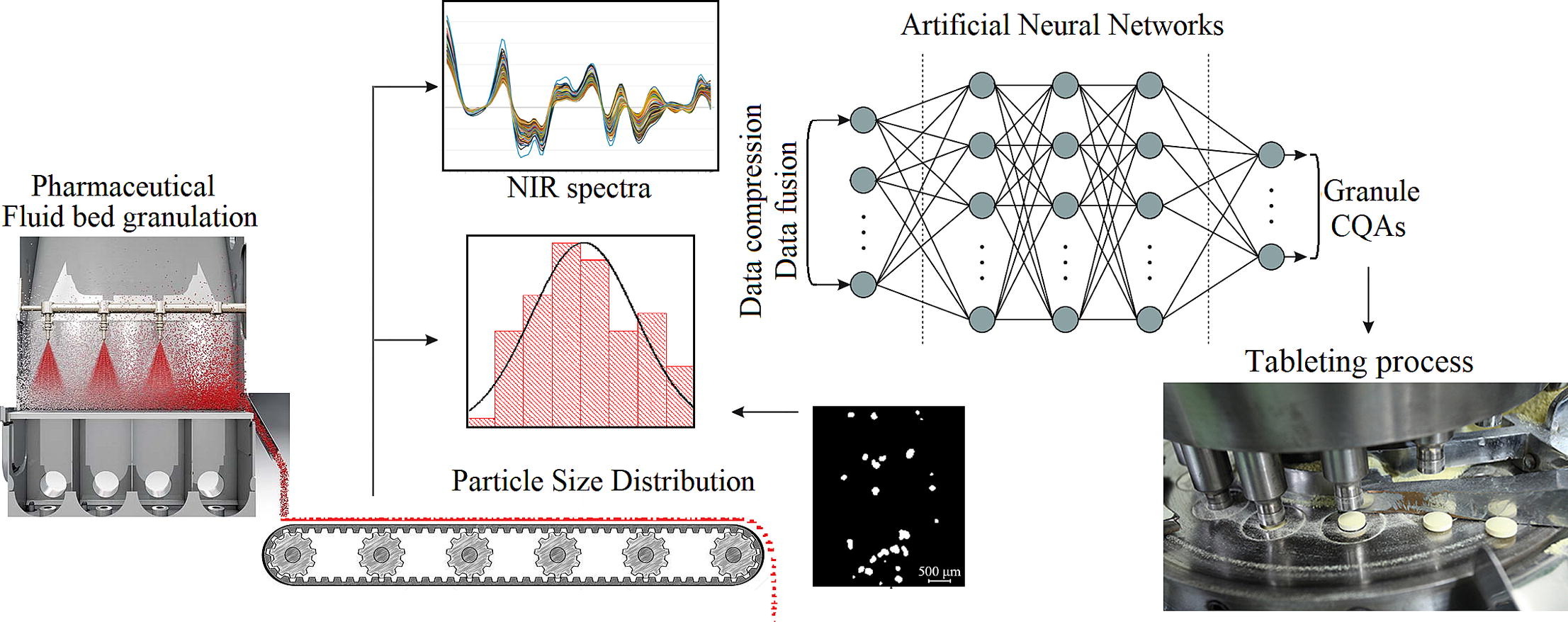
In this work, the feasibility of implementing a process analytical technology (PAT) platform consisting of Near Infrared Spectroscopy (NIR) and particle size distribution (PSD) analysis was evaluated for the prediction of granule downstream processability. A Design of Experiments-based calibration set was prepared using a fluid bed melt granulation process by varying the binder content, granulation time, and granulation temperature. The granule samples were characterized using PAT tools and a compaction simulator in the 100-500kg load range. Comparing the systematic variability in NIR and PSD data, their complementarity was demonstrated by identifying joint and unique sources of variation.
Highlights
- NIR and granule size distribution data were fused to predict tableting properties.
- ANN-based modeling offered an improved performance compared to PLS.
- Tabletability and stress values during tablet detachment/ejection were predicted.
These particularities of the data explained some differences in the performance of individual models. Regarding the fusion of data sources, the input data structure for partial least squares (PLS) based models did not significantly impact the predictive performance, as the root mean squared error of prediction (RMSEP) values were similar. Comparing PLS and artificial neural network (ANN) models, it was observed that the ANNs systematically provided superior model performance. For example, the best tensile strength, ejection stress, and detachment stress prediction with ANN resulted in an RMSEP of 0.119, 0.256, and 0.293 as opposed to the 0.180, 0.395, and 0.430 RMSEPs of the PLS models, respectively. Finally, the robustness of the developed models was assessed.
Download the full research paper as PDF (Pre-Proof) here: Development of a PAT platform for the prediction of granule tableting properties
or read it here
Materials
Clopidogrel hydrogen sulphate, form II (MSN Laboratories Ltd, Telangana, India), Mannitol 35 (Pearlitol 50C) (Roquette Frères, Lestrem, France), Macrogol 8000 (Dow Chemical Company, Hahnville, LA, USA), Cellulose, microcrystalline M103D+ (Mingtai Chemical Co., Ltd., Taoyuan City, Taiwan), Low-substituted hydroxypropyl-cellulose (L-HPC, LH-11) (Shin-Etsu Chemical Co., Ltd., Tokyo, Japan). All the materials used in this study were of pharmaceutical grade.
Tibor Casian, Brigitta Nagy, Cristiana Lazurca, Victor Marcu, Erzsébet Orsolya Tőkés, Éva Katalin Kelemen, Katalin Zöldi, Radu Oprean, Zsombor Kristóf Nagy, Ioan Tomuta, Béla Kovács, Development of a PAT platform for the prediction of granule tableting properties, International Journal of Pharmaceutics, 2023, 123610, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123610.
See other recent articles on PAT topics:

