Pediatric Formulations Developed by Extrusion-Based 3D Printing: From Past Discoveries to Future Prospects
Abstract
Introduction
Everything began in the 1980s with the invention and the development of the first 3D printing technologies such as selective laser sintering (SLS) and fused deposition modeling (FDM) [1,2,3]. More specifically, in 1989, fused deposition modeling (FDM) was originally invented and patented by Stratasys co-founder Scott Crump [4]. The rapid expansion of 3D printing technology has given a new edge to introduce innovative medical devices and customized drug delivery systems.
Most drug products are typically manufactured in large quantities using conventional methods that involve large-scale processes and equipment and long production times. The pharmaceutical industry is interested in using this 3D printing technology to overcome conventional mass manufacturing in order to develop personalized pharmaceutical therapy [5].
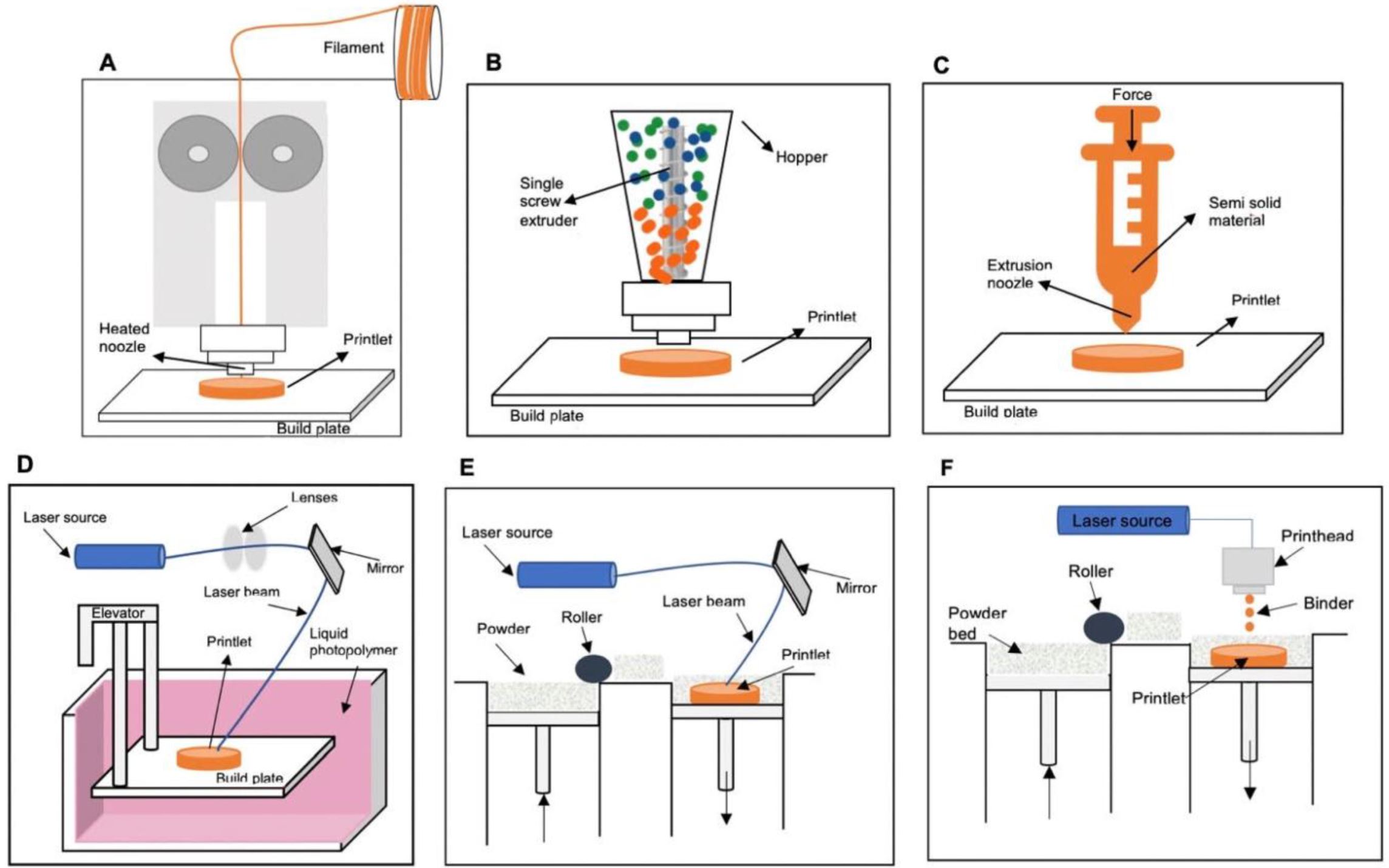
Three-dimensional printing is a form of additive manufacturing in which a three-dimensional object is built by depositing building materials in successive layers according to a predesigned three-dimensional geometric structure. Three-dimensional printing of pharmaceuticals is a unique approach that allows for the manufacture of solid drug products in various shapes, geometric designs, strengths and spatial distributions of the active pharmaceutical ingredients. Three-dimensional structures ranging from simple one-compartmental designs to complex multi-compartmental designs can be produced. The release profile of the active ingredients from these complex 3D drug products can be tailored to meet the needs of specific patients [6].
Moreover, three-dimensional printing (3DP) technology allows the adjustment of release kinetics and of individual doses, providing significant benefits for pediatric patients. Individual release characteristics may be required in this patient group in addition to dose adjustment. For instance, children may need special or smaller doses beyond what is conventionally available, or they may need unique dosage forms other than the standard pill, which can be difficult to swallow. Older adults may have various physiological or metabolic conditions due to certain illnesses or resulting from taking multiple medications, which may require alterations in doses or dosage forms on an ongoing basis. Moreover, it may be possible to combine certain medications into one “polypill” using 3D designs and 3D printing processes, which would be especially useful for those who take multiple medications every day [7,8].
All of these factors have also increased interest in 3DP technology in the pharmaceutical industry for its wide range of applications and versatility. Furthermore, the commercial feasibility of the Spritam® 3DP tablet, an antiepileptic drug developed by Aprecia Pharmaceuticals (Blue Ash, OH, USA) and approved by the FDA in August 2015, indicates the potential for the commercial feasibility of manufacturing fast-dissolving oral tablets with high drug-loading profiles using ZipDose additive manufacturing technology. It is dispersed in the mouth with a very small amount of water in less than 10 s, making it very easy to use in populations of disadvantaged patients (e.g., pediatric patients or elderly patients) [9].
On the other hand, 3DP comes with the burden of challenges such as the requirements for excipients, the development of printing software and instrumentation, optimizing the mechanical properties of products and the regulatory landscape [10].
Unlike conventional methods of manufacturing that are required for so-called “blockbuster drugs” and create billions of tablets per year for global supply, 3D printing is already, or will soon be, well suited to supply orphan or smaller oncology indications, where only millions of tablets are required per year.
One of the purposes of this review is to summarize the discoveries made in recent years using extrusion-based 3D printing techniques to develop pediatric pharmaceutical forms. The other is to highlight the potential of 3D printing in revolutionizing conventional manufacturing toward small batch manufacturing (flexible and tailored pharmaceutical forms) and its advantages, such as safer and more effective treatments.
Download the full article as PDF here: Pediatric Formulations Developed by Extrusion-Based 3D Printing
or read it here
Ianno, V.; Vurpillot, S.; Prillieux, S.; Espeau, P. Pediatric Formulations Developed by Extrusion-Based 3D Printing: From Past Discoveries to Future Prospects. Pharmaceutics 2024, 16, 441. https://doi.org/10.3390/pharmaceutics16040441
Are you interested in our webinar:
“Rethinking the development of controlled release formulations and manufacturing processes”
Date: 30th of April, Time: 3:00 pm (Amsterdam, Berlin)


