Supersaturable diacyl phospholipid dispersion for improving oral bioavailability of brick dust molecule: A case study of Aprepitant
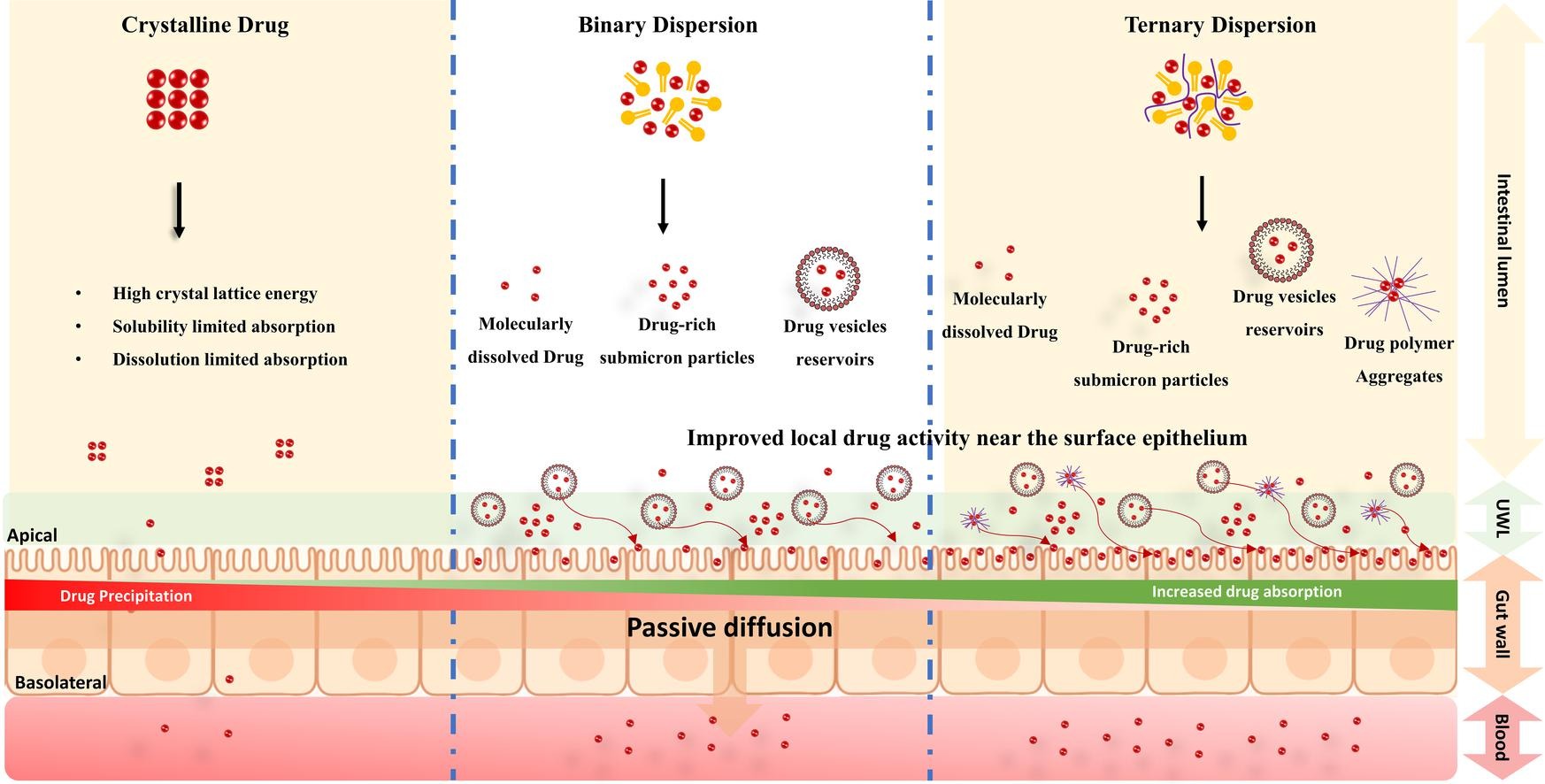
This study aims to investigate the potential use of polymer inclusion in the phospholipid-based solid dispersion approach for augmenting the biopharmaceutical performance of Aprepitant (APT). Initially, different polymers were screened using the microarray plate method to assess their ability to inhibit drug precipitation in the supersaturated solution and HPMCAS outperformed the others. Later, the binary (BD) and ternary (TD) phospholipid dispersions were prepared using the co-solvent evaporation method. Solid-state characterization was performed using SEM and PXRD to examine the physical properties, while molecular interactions were probed through FTIR and NMR analysis. In vitro dissolution studies were performed in both fasted and fed state biorelevant media.
The results demonstrated a substantial increase in drug release from BD and TD, approximately 4.8 and 9.9 times higher compared to crystalline APT in FaSSIF. Notably, TD also showed a lowered dissolution difference between fed and fasted states in comparison to crystalline APT, indicating a reduction in the positive food effect of APT. Moreover, we assessed the impact of polymer inclusion on permeation under in vitro biomimetic conditions. In comparison with the crystalline APT suspension, both BD and TD demonstrated approximately 3.3 times and 14 times higher steady-state flux (Jss values), respectively. This can be ascribed to the supersaturation and presence of drug-rich submicron particles (nanodroplets) along with the multiple aggregates of drug with phospholipids and polymer in the donor compartment, consequently resulting in a more substantial driving force for passive diffusion.
Lastly, in vivo pharmacokinetic evaluation demonstrated the enhanced absorption of both TD and BD over the free drug suspension in the fasted state. This enhancement was evident through a 2.1-fold and 1.3-fold increase in Cmax and a 2.3-fold and 1.4-fold increase in AUC0-t, respectively. Overall, these findings emphasize the potential of polymer-based phospholipid dispersion in enhancing the overall biopharmaceutical performance of APT.
Read more on phospholipid dispersion for improving oral bioavailability
Materials
APT was kindly donated as a gift sample by Hetero Drugs Ltd. (Hyderabad, India). The various phospholipids such as Lipoid® S 100 (Phosphatidylcholine 100 %), Phospholipon® 90H (Hydrogenated Phosphatidylcholine ≥ 94 %), and Lipoid® SPC (Hydrogenated phosphatidylcholine ≥ 90 %) were provided as gift samples by Lipoid GmbH (Ludwigshafen, Germany) and used without further purification. HPMCAS-MF was purchased from Shin-Etsu Chemical Co. Ltd. (Mumbai, India).
Ajay Sanjay Lale, Arvind Sirvi, Shubham Debaje, Sadhana Patil, Abhay T. Sangamwar, Supersaturable diacyl phospholipid dispersion for improving oral bioavailability of brick dust molecule: A case study of Aprepitant,
European Journal of Pharmaceutics and Biopharmaceutics, Volume 197, 2024, 114241, ISSN 0939-6411, https://doi.org/10.1016/j.ejpb.2024.114241.
Read also our introduction article on Orally Disintegrating Tablets (ODTs) here:


