Use of a Platform Formulation Technology to De-Risk Solid-State Variation in Drug Development
Active pharmaceutical ingredients (APIs) can exist in multiple crystal forms, known as polymorphs, that are chemically identical. Polymorphs of an API can appear over the course of development and lead to deviations across batches affecting stability, tableting properties, flowability, solubility, and release kinetics, and may ultimately result in a change in processability and drug (product) performance. These changes must be identified and addressed and as such, can have a significant impact on the success of the program, project timelines, and the investment of resources. While crystal structure prediction techniques can be used to identify possible polymorphs, their stability and morphological differences, especially the presence of unstable polymorphs, must be addressed to ensure product efficacy and safety.
A study by Cordeiro, et al., determined whether different formulation strategies could be used to stabilize molecules such as menthol which have a high polymorphism risk and a high risk of recrystallizing from the amorphous form.1 Results of the study demonstrated a robust stabilization of menthol molecules by absorption onto the surface of a mesoporous silica carrier.
This white paper describes use of mesoporous silica as a porous carrier formulation technology to stabilize unstable polymorphs and to optimize solid state properties. The strategy can be used across a broad range of APIs and can thus serve as a platform technology for consistent particle properties leading to more efficient formulation development processes.
Mesoporous Silica as a Carrier for Unstable Polymorphs
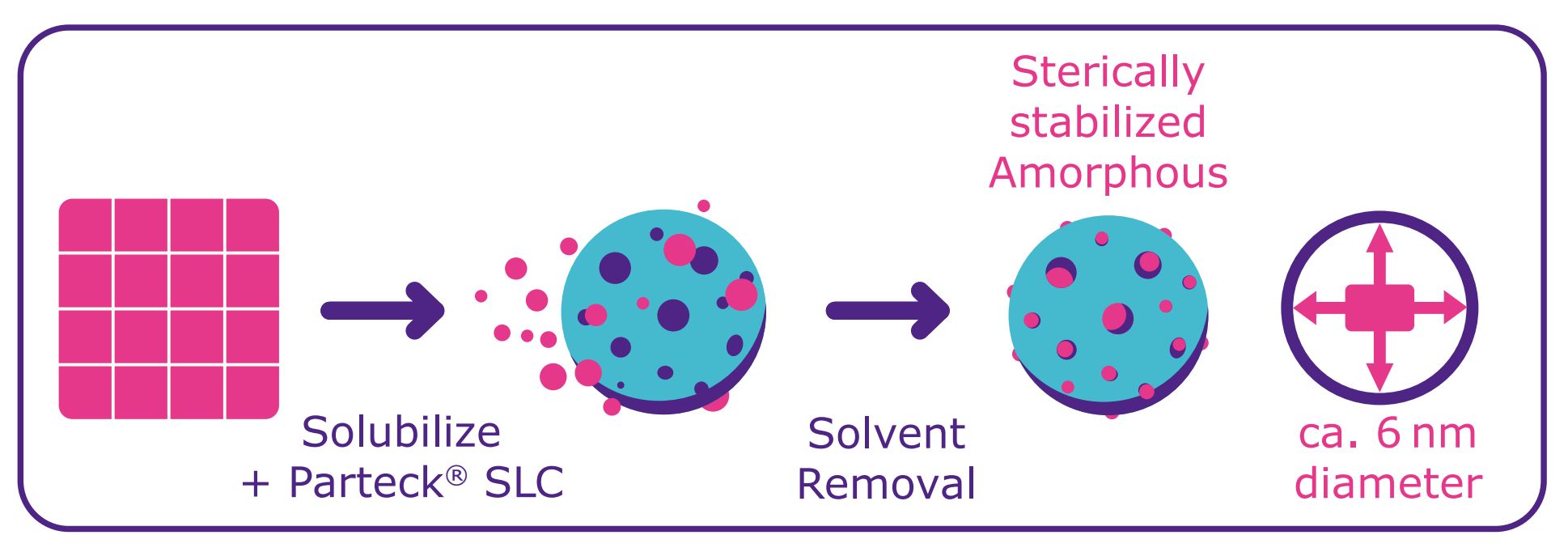
Parteck® SLC excipient is a mesoporous silica that can serve as a porous solid carrier for formulations of APIs in which unstable polymorphs may be present. The excipient is based on silicon dioxide and is generally regarded as safe (GRAS) by regulatory authorities. The silica particles have a D50 of 17–25 µm with a bulk density of 0.32 g/mL, which can be increased when loading an API within the pore structure. Parteck® SLC excipient by Merck offers a very large surface area of 500 m2/g which is essential for performance as a carrier and has a very small average size of approximately 6 nm disordered pores which are essential for sterically stabilizing the amorphous form of APIs (Table 1).
Table 1.
Properties of Parteck® SLC mesoporous silica.
Chemical formula: SiO2
Pharmacopoeial monograph: Silicon Dioxide (USP) and Silica,
colloidal hydrated (Ph. Eur.)
Regulatory status: Generally Regarded As Safe (GRAS)*
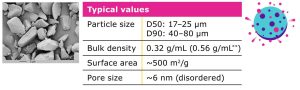
* By the U.S. Food and Drug Administration
** Parteck® SLC loaded with 30% ibuprofen.
Density is increased upon loading with API.
For lab-scale loadings of 1–200 g of API onto Parteck® SLC mesoporous silica, an impregnation or suspension method with simple laboratory equipment can be used; no additional capital investment is needed. For the impregnation method, the Parteck® SLC excipient is placed in a beaker and the API, dissolved in an organic solvent, is dropped onto the carrier using a cannula. A suspension method can also be used in which the API in an organic solvent is mixed with the carrier and stirred. The organic solvent is then removed via evaporation. Loadings of up to several hundred kilograms can be accomplished by simply transferring the process to a larger scale and slowly applying the API solution to the carrier material to successfully stabilize the amorphous form within the mesoporous system.
Stabilization Mechanism
The API is stabilized in the amorphous form due to its interaction with the carrier via the large surface area (Figure 1). The risk of detecting another polymorph during the development phase is reduced because the amorphous stabilization prevents crystallization.
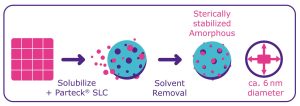
Stabilization of the amorphous form of poorly soluble molecules using
Parteck® SLC excipient.
To demonstrate the stabilization mechanism of mesoporous silica, fenofibrate, a poorly soluble molecule, was loaded onto Parteck® SLC excipient using the impregnation method; fenofibrate was dissolved in acetone. The differential scanning calorimetry (DSC) graph in Figure 2 shows that the fenofibrate was present in its amorphous state when loaded on the Parteck® SLC excipient at 30%. As a reference, the crystalline API was included in the analysis. If the API is present in its crystalline solid-state, a melting endotherm occurs, as indicated by a downwards spike; if the API is within its
amorphous form, this spike does not occur.
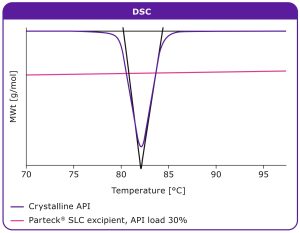
DSC results show that fenofibrate is stabilized in its amorphous form
when loaded onto Parteck® SLC excipient, in comparison to pure
crystalline API.
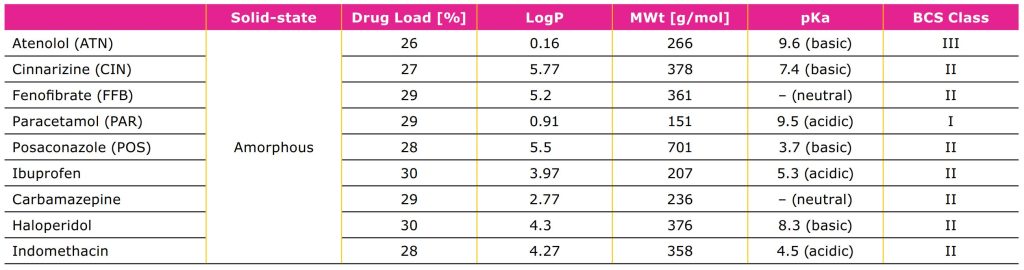
A range of APIs with different chemistries, hydrophobicities, and molecular weights have been stabilized in the amorphous form by loading onto the Parteck® SLC excipient (Table 2). Parteck® SLC excipient is also a best-in-class technology for stabilizing amorphous forms of molecules which tend to recrystallize (poor glass former; GFA-I molecules such as carbamazepine) and are particularly difficult to formulate.2
Table 2.
Examples of poorly soluble APIs of different chemistry and physicochemical properties which were successfully stabilized in their amorphous form by loading onto Parteck® SLC silica.
Improving Galenical and Particle Properties
As shown in Table 2, APIs with different chemistries
and physiochemical properties can be stabilized in
their amorphous state using Parteck® SLC excipient.
The following studies investigated the effect of
loading various APIs onto Parteck® SLC excipient on
critical properties of the solid formulation process
such as particle size, flowability, and compression.
Results demonstrated that the technology can be
used across different APIs to improve solid state
performance. Consistent particle properties opens
up the opportunity to use Parteck® SLC excipient as
a platform technology in formulation development to
improve process performance, formulation process
efficiency, and drug performance.
A series of different APIs were loaded onto Parteck®
SLC excipient and the resulting particle size determined
to evaluate whether the excipient can be used
successfully independent of the API and in the same
formulation composition. The results showed that a
similar medium particle size was achieved, regardless
of the API, which is a prerequisite for use of the
technology as general formulation approach (Figure 3).
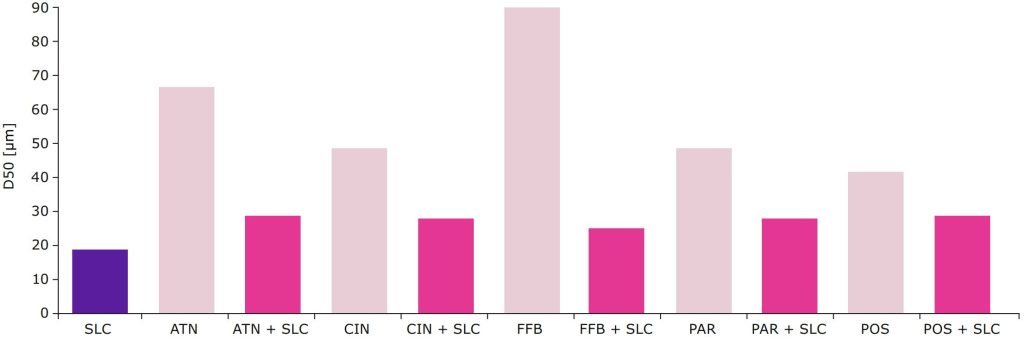
Particle size distribution of model APIs atenolol (ATN), cinnarizine (CIN), fenofibrate (FFB), paracetamol (PAR), and posaconazole (POS) prior to
and after loading onto Parteck® SLC mesoporous silica.
The flowability of a range of diverse small molecule APIs was improved compared to the pure API after loading onto the Parteck® SLC excipient carrier (Figure 4). The consistency in flowability indicates that the properties for final formulation are similar across APIs, reinforcing the potential of this carrier technology to be widely applicable.
See the full White Paper on “Use of a Platform Formulation Technology to De-Risk Solid-State Variation in Drug Development” here
(click the picture to download the brochure)
Source: Merck White Paper “Use of a Platform Formulation Technology to De-Risk Solid-State Variation in Drug Development”
Do you need more information or a sample of excipients by Merck?


