Comprehensive Evaluation of Polymer Types and Ratios in Spray-Dried Dispersions: Compaction, Dissolution, and Physical Stability
Abstract
Amorphous solid dispersion (ASD) is a well-established strategy for enhancing the solubility and bioavailability of poorly soluble drugs. A significant portion of ASD products are in tablet form. However, the influence of common polymers and drug loading on the manufacturability of ASD tablets remains underexplored. This study focuses on investigating spray-dried ASDs from a tableting perspective by evaluating their physiochemical and mechanical properties. Itraconazole (ITZ) and indomethacin (IND), at the drug loadings ranging from 10% to 50%, were prepared with two polymers, hydroxypropyl methylcellulose acetate succinate (HPMCAS) and polyvinylpyrrolidone (PVP), serving as representative systems. Our findings revealed that increasing the drug loading resulted in a decreased surface area in ITZ-HPMCAS, IND-HPMCAS, and IND-PVP ASDs. However, this trend was not observed in ITZ-PVP dispersions, possibly due to the morphological disparities. Compaction results demonstrated that tabletability improved with decreasing drug loadings, except for ITZ-PVP dispersions. A partial least square analysis underscored particle surface area as the key factor influencing the tensile strength of ASD tablets. Additionally, our study disclosed that ITZ-PVP ASDs exhibited the worst release profiles and stability performance. The comprehensive journey from characterizing ASD particles to analyzing their compaction behavior and investigating drug release and physical stability offered profound insights into the attributes crucial for the downstream processing of amorphous pharmaceuticals.
Introduction
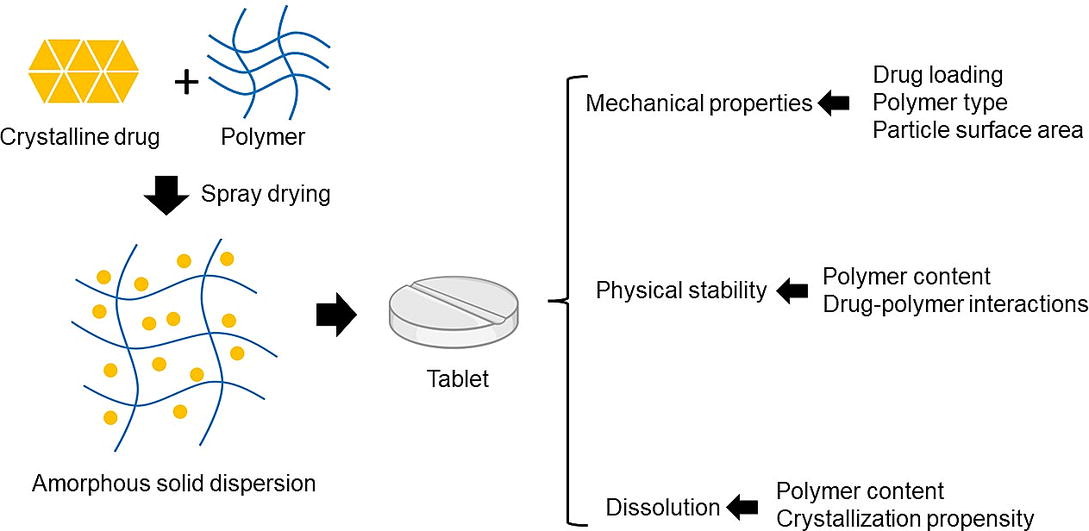
A considerable number of new chemical entities in the development pipeline face challenges due to their low aqueous solubility, which hinders their ability to dissolve adequately in the gastrointestinal tract for effective drug absorption (Kalepu and Nekkanti, 2015). To overcome these solubility limitations, amorphous solid dispersions (ASDs) have emerged as a promising approach (Goldberg et al., 1965). Spray drying, a solvent-based, continuous, and scalable drying process, has been extensively utilized in particle engineering and ASD production. However, the spray dried ASD needs to be further processed into a final dosage form for proper administration.
Tablets are widely used as the dosage form in oral drug delivery due to their advantages in terms of patient compliance, scalability, and cost-effectiveness. This has made them the preferred option for over half of the marketed ASD products, with a notable portion of them prepared using spray drying technology (Jermain et al., 2018, Pandi, 2020). The spray-dried dispersions usually obtain a porous structure, which is a desirable attribute for high compressibility. However, their small particle size and low bulk density can hinder powder flowability (Honick, 2020). In the pharmaceutical context, ‘manufacturability’ refers to the ability to produce tablets efficiently on a large scale while adhering to quality and regulatory standards. It is important to note that the molecular-to-bulk properties of ASDs can significantly impact tablet manufacturability. These properties encompass aspects like powder flow, tableting behavior, overall performance, and stability, all of which are critical for formulation and process development (Paudel, 2013). Despite its importance, there is a noticeable lack of comprehensive studies delving into the manufacturability of ASDs. This study aims to bridge this gap by investigating the physicochemical and mechanical properties of model spray-dried dispersions. Our specific focus is on understanding how the choice of polymer and drug loading levels influence the compaction performance of these dispersions.
In ASDs, research revealed that higher drug loading can adversely affect physical stability and dissolution performance (Pandi, 2020, Deac, 2022). From a mechanical perspective, previous studies have demonstrated that amorphous films containing acetaminophen/poly(1-vinylpyrrolidone-co-vinyl acetate) (PVPVA) and clotrimazole/PVPVA exhibit an increase in nanoindentation hardness with increased drug loading. This increase is attributed to the anti-plasticization effect of the polymer caused by the presence of drug molecules. However, it’s important to note that the drug loading required to achieve maximum hardness varies depending on the specific system (Patel, 2017, Lamm, 2012). Conversely, further compaction studies have shown contrasting behaviors in tablet tensile strength for different drug-polymer systems and experimental conditions. In the case of acetaminophen/PVPVA ASDs produced through hot melt extrusion, tablet tensile strength declined with increasing drug concentration (from 0% to 15%) at a given porosity. This decline was primarily due to lower bonding strength between ASD particles at higher drug loading (Patel, 2017). On the other hand, another study revealed that the tensile strength of itraconazole/PVPVA spray-dried dispersions reached its maximum at 40–60% drug loading but decreased as the drug loading further increased under 33% RH conditions. This decrease in tensile strength at high polymer content was associated with the hydrophilic nature of PVPVA. At lower drug loading levels, the absorbed water had a plasticizing effect, leading to a reduction in the tensile strength of the spray-dried dispersions (Zhang, 2022). It is noteworthy that during the compaction study, processing parameters, specifically compression pressure and speed, play pivotal roles in shaping the characteristics of the resulting tablet. For example, compaction speed can exert a notable influence on tablet quality. High compaction speeds have been observed to induce lamination issues in tablets formulated with spray-dried ASDs (Honick, 2020). Furthermore, the inclusion of external excipients, such as fillers and disintegrants, can introduce instability into the ASD system. For example, research has shown that commonly used lubricants, like magnesium stearate, could act as a nucleation-promoting agent and induce recrystallization of amorphous drugs (Démuth, 2016). Additionally, the presence of excipients can alter the compaction behavior of ASDs. As we delve into understanding the processing of ASDs, it’s crucial to initiate our journey with the neat ASD as the starting point. From there, we can progressively extend our studies to more complex formulation systems.
The primary objective of this study is to enhance our understanding of the manufacturability of neat ASDs. This comprehensive endeavor encompasses the assessment of various aspects, including the properties of bulk powders, mechanical attributes, performance, and physical stability within model systems. Through these comprehensive evaluations, we aspire to gain valuable insights that will ultimately enable us to better comprehend the formulation and manufacturing processes necessary for the development of more efficient oral drug delivery systems. In our work, itraconazole (ITZ) (aqueous solubility = 1 – 5 ng/mL at 25 °C, log P = 5.7, pKa = 3.7 (Information, N.C.f.B., PubChem Compound Summary for CID 55283, Itraconazole. Retrieved August 30, 2021) and indomethacin (IND) (aqueous solubility = 0.94 mg/L at 25 °C, log P = 3.4, pKa = 4.5 (Information, N.C.f.B., PubChem Compound Summary for CID 3715, Indomethacin. Retrieved August 30, 2021) are used as model drugs, encompassing three drug ratios: 10%, 30%, and 50%. Both drugs fall within BCS Class 2, characterized by solubility-limited oral absorption (Figure 1). Hypromellose acetate succinate (HPMCAS) L grade and PVP are employed as model carriers. HPMCAS, a cellulosic polymer extensively used in ASDs (Deshpande, 2018), is known for its diverse degrees of substitution for acetyl and succinyl groups within the polymer structure (Table S1). HPMCAS L grade represents a polymer with a high ratio of succinyl substitution to acetyl substitution (S/A ratio), yielding elevated hydrophilicity. Therefore, the rapid release of the drug under neutral pH conditions was most evident in ASDs formulated with HPMCAS L grade (Tanno, 2004, Yu, 2022). In terms of physical stability, HPMCAS achieves ASD stabilization through hydrophobic interactions, such as those between the acetyl groups of HPMCAS and the drug’s hydrophobic components (Chen, 2018, Yu, 2022). PVP, one of the most prevalent hydrophilic polymers, was so incorporated in our study (Taylor and Zografi, 1997). Compaction studies were conducted using a compaction simulator.
Read more here
Materials
The model drugs, ITZ USP grade (lot # 1812200066) and IND USP grade (lot # 7QZFJBG), were purchased from Letco Medical (Wayne, PA) and TCI America, Inc. (Portland, OR), respectively. PVP K30 (Tg: 163 °C, lot # 15277947G0) was obtained from BASF Corporation (Ledgewood, NJ). HPMCAS L grade (Tg: 119 °C, lot # 55G-710002) was purchased from Ashland Global Specialty Chemicals Inc. (Covington, KY). All organic solvents used for the spray drying process were ACS grade.
Dongyue Yu, Haichen Nie, Stephen W. Hoeg, Comprehensive Evaluation of Polymer Types and Ratios in Spray-Dried Dispersions: Compaction, Dissolution, and Physical Stability, International Journal of Pharmaceutics, 2023, 123674, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123674.
Watch our free webinar about “Spray drying with Shin-Etsu, ProCepT and Xedev” here:


