Polymeric micelles for cutaneous delivery of the hedgehog pathway inhibitor TAK-441: formulation development and cutaneous biodistribution in porcine and human skin
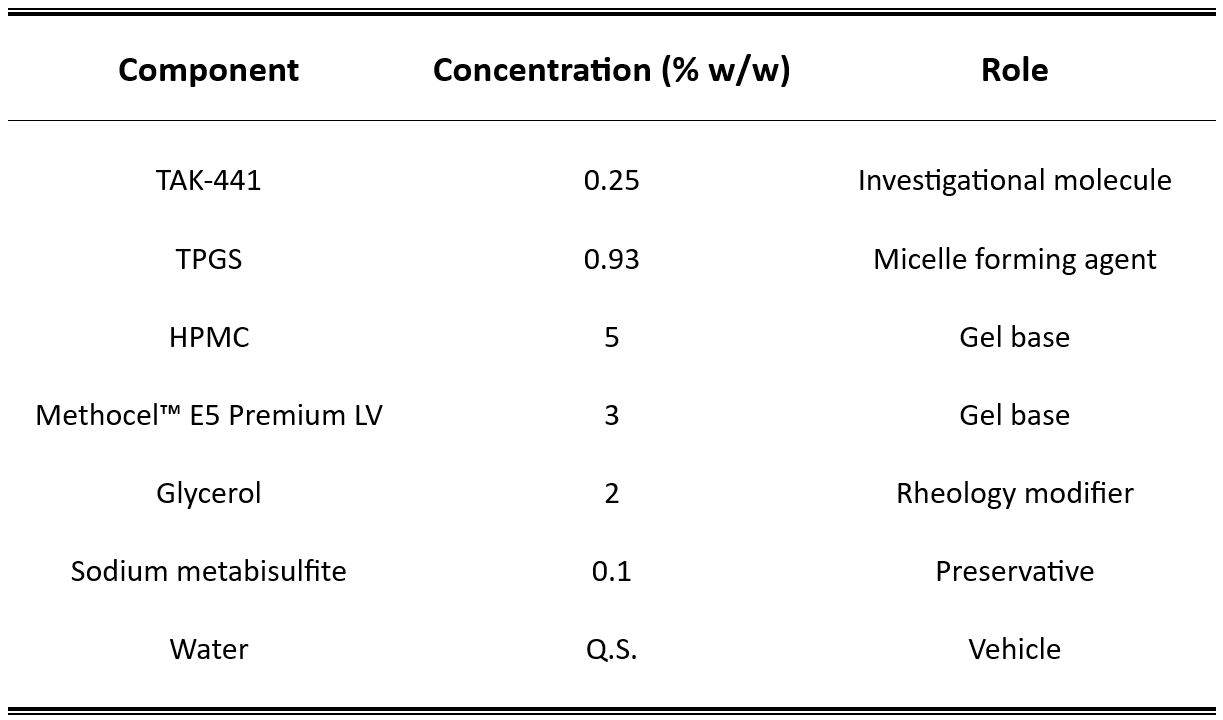
TAK-441 is a potent inhibitor of the hedgehog pathway (IC50 4.4 nM) developed for the treatment of basal cell carcinoma that is active against the vismodegib-resistant Smoothened receptor D473H mutant. The objective of this study was to develop a micelle-based formulation of TAK-441 using D-α-Tocopherol polyethylene glycol 1000 succinate (TPGS) and to investigate its cutaneous delivery and biodistribution. The micelles were prepared using solvent evaporation and incorporation of TAK-441 in the TPGS micelles increased aqueous solubility ∼40-fold. The optimal formulation, a 3% HPMC hydrogel of TAK-441 loaded TPGS micelles, retained ∼92% of the initial TAK-441 content (2.5 mgTAK-441/g) after storage at 4 °C for 6 months.
Finite dose experiments using human skin demonstrated that this formulation resulted in significantly greater cutaneous deposition of TAK-441 after 12 h than a non-micelle control formulation, (0.40 ± 0.11 µg/cm2 and 0.05 ± 0.02 µg/cm2, respectively) – no transdermal permeation was observed. The cutaneous biodistribution profile demonstrated that TAK-441 was predominantly delivered to the viable epidermis and upper dermis. Delivery from the HPMC hydrogel formulation resulted in TAK-441 epidermal concentrations that were several thousand-fold higher than the IC50, with almost negligible transdermal permeation, thereby decreasing the risk of systemic side effects in vivo.
Download the full article as PDF here Polymeric micelles for cutaneous delivery of the hedgehog pathway inhibitor TAK-441: formulation development and cutaneous biodistribution in porcine and human skin
or read it here
Materials
TAK-441 was kindly provided by Takeda Pharmaceutical Company Ltd, Japan. D-α-Tocopherol polyethylene glycol 1000 succinate (TPGS), formic acid (MS grade), isopentane, and Dulbecco’s phosphate-buffered saline (DPBS), hydroxypropyl methylcellulose (HPMC, ∼26 kDa; methoxyl content 19-24 % and hydroxypropoxyl content 7-12 %) were purchased from Sigma-Aldrich (Buchs, Switzerland). Low molecular weight HPMC – Methocel™ E5 Premium LV (∼10 kDa; methoxyl content 28-30 % and hydroxypropoxyl content 7-12 %) was procured from Dow Chemicals (Horgen, Switzerland). Hydroxypropyl cellulose (Klucel™ MF Pharm, HPC; M.W. ∼ 850 kDa) and glycerol were purchased from Hänseler AG (Herisau, Switzerland). Bovine serum albumin (BSA) was purchased from Axon Lab (Baden-Dättwil, Switzerland). Acetone (analytical grade) and Nile Red dye were obtained from Acros Organics (Geel, Belgium). Methanol and acetonitrile (LC-MS grade) were purchased from Fisher Scientific (Reinach, Switzerland). PTFE membrane filters (0.22 μm), Amicon Ultra 0.5 mL (5 kDa) filtration units were purchased from VWR (Nyon, Switzerland). Ultrapure water (Millipore Milli-Q Gard 1 Purification Pack resistivity >18 MΩ.cm; Zug, Switzerland) was used for formulation development and analysis. All other chemicals were at least of analytical grade.
Aditya R. Darade, Maria Lapteva, Vincent Ling, Yogeshvar N. Kalia, Polymeric micelles for cutaneous delivery of the hedgehog pathway inhibitor TAK-441: formulation development and cutaneous biodistribution in porcine and human skin, International Journal of Pharmaceutics, 2023, 123349, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123349.

