Effect of Precirol ATO5 concentration and twin-screw melt granulation temperature on the physical properties of ascorbic acid granules
SUMMARY
The pharmaceutical industry has a growing interest in continuous manufacturing, particularly for high-volume dosage forms such as solid oral tablets. This is driven by the obvious benefits such as small footprint, batch size flexibility, the relative simplicity of scale-up and process control. Tablets are the most popular solid dosage form, while batch granulation is one of the most often used technologies in tablet manufacturing. Using ascorbic acid as a model, we show the possibility of producing granules and tablets with a sustained-release profile using three technological operations: mixing, continuous melt-granulation, and tabletting.
INTRODUCTION
Currently, sustained-release hydrophilic matrix tablets of ascorbic acid (as 500 mg Vitamin C from Boots, UK) are marketed as a way to decrease the detrimental effects of the active ingredient on gastrointestinal tissue. In this work, we demonstrate the use of HME as a way of continuously manufacturing SR granules for subsequent tableting purposes.
The aim of this investigation was to prepare sustained-release tablets of ascorbic acid by continuous twin-screw melt granulation and to assess the effect of the concentration of a low-melting insoluble lipid matrix former (Precirol ATO5) and twin-screw melt granulation temperature on the properties of granules and tablets.
MATERIALS AND METHODS
L-ascorbic acid (SigmaAldrich, UK) and Precirol ATO5 (Gattefossé, France) were used as an active pharmaceutical ingredient (API) and low-melting insoluble matrix-former, respectively. Ingredients were mixed and melt-granulated using a horizontal 10mm benchtop co-rotating twin-screw extruder (L/D 20:1; Microlab; Rondol Tech. Ltd., France) equipped with conveying elements only at a feeding rate of 1.5 g/min, screw speed of 120 rpm, and different processing temperatures (Table 1). Resultant granules without additional processing were compressed into flat-faced 11mm diameter tablets at 60 kN using a hydraulic press and the hardness of tablets was tested (TBH 125; ERWEKA
GmbH., Germany).
Thermogravimetric analysis (TGA; Q50, TA Instruments, UK), differential scanning calorimetry (DSC) with integrated analysis software (Version 4.5A; TA Instruments, USA), powder X-ray diffraction (pXRD; Mini-Flex II; Rigaku Corp., Japan) scanning electron microscopy (SEM; TM3030; Hitachi High-Technologies Corp., Japan), particle size
distribution (PSD; sieving method), powder flowability with gravitational funnel (10mm hol size) method, angle of repose (AoR), bulk and tapped density, as well as Hausner ratio (HR), were utilised for characterisation purposes.
RESULTS AND DISCUSSION
Ascorbic acid (D50=85.9µm) and Precirol ATO5 (D50=51.8µm) showed thermal stability up to 200℃ (TGA). Ascorbic acid and Precirol ATO5 demonstrated melting peaks (Mp) of 195.0 and 71.4℃ after the first heating cycle (DSC). Precirol ATO5 demonstrated crystallization upon cooling with a crystallization peak at 51.8℃ and a decreased
Mp of 61.0℃ during the second heating cycle (DSC). Due to the relatively low granulation temperature (Table 1), the solid-state of ascorbic acid was not affected by melt granulation (confirmed with DSC and pXRD) [1].
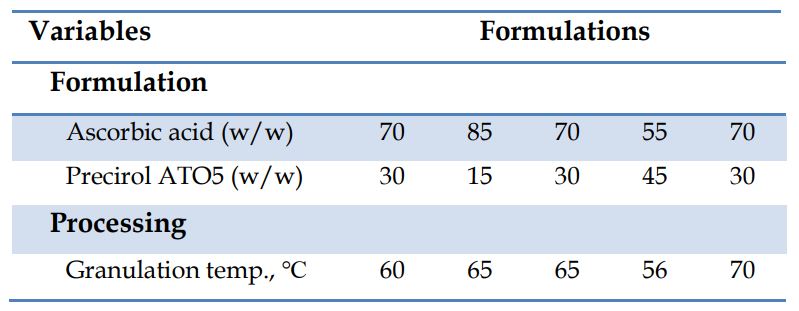
The particle size of granules increased along with the increased granulation temperature and Precirol ATO5 concentration (Fig. 1A) which was additionally demonstrated with SEM [1]. Similarly, flowability increased (Fig. 1B) and AoR (Fig. 2A) decreased as a function of increases in granulation temperature and Precirol ATO5 concentration.
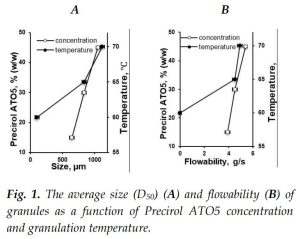
The HR values were in agreement with flowability. The highest HR (1.30) value was observed at the lowest granulation temperature, while for the rest of the formulations, the HR was similar (1.15-1.18) and suggested good flowability.
Precirol ATO5 was shown to have excellent ability as a lubricant and no additional lubricant, was required. The highest tablet hardness was obtained for the formulation processed at a higher temperature, while the difference between the rest of the formulations was not as significant (Fig. 2B). Among investigated formulations, the formulation prepared at 70℃ demonstrated the closest drug release profile to the marketed formulation [1].
CONCLUSIONS
Precirol ATO5 concentration and twin-screw melt granulation temperature had a significant influence on the properties of granules and tablets. Generally, at the chosen extrusion settings, an increase of Precirol ATO5 concentration from 15 to 30% (w/w) and processing temperature from 60 to 70℃ improved the performance of granules for tabletting
purposes.
Download the full article as PDF here Effect of Precirol ATO5 concentration and twin-screw melt granulation temperature on the physical properties of ascorbic acid granules
or read it here
Mohylyuk, V., Ding, Y., Andrews, G.P., 2022. Effect of Precirol ATO5 concentration and twin-screw melt granulation temperature on the ascorbic acid release. PBP (Rotterdam). DOI: 10.13140/RG.2.2.11580.31368

