pH-dependent pressure-sensitive colonic capsules for the delivery of aqueous bacterial suspensions
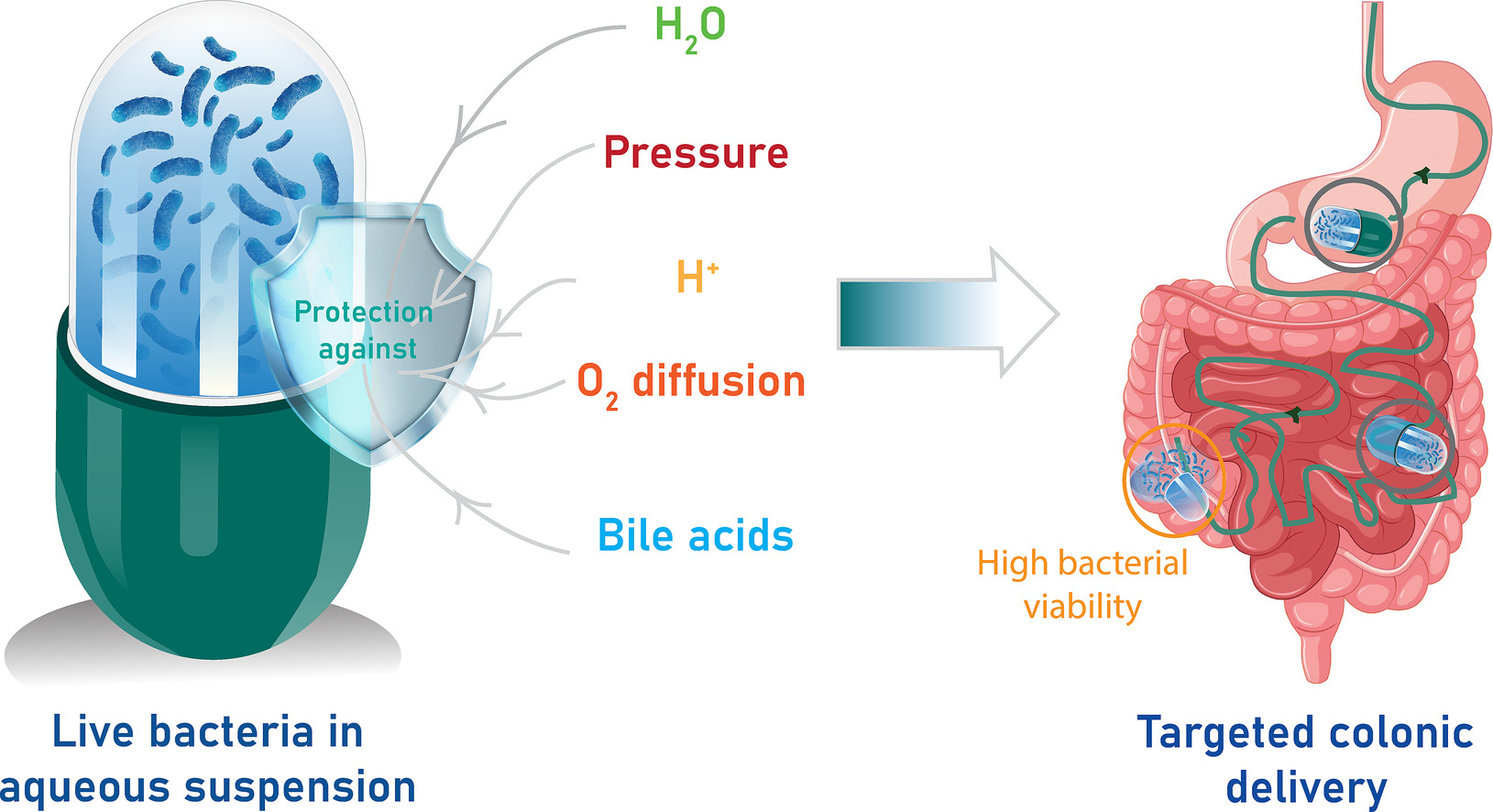
Microbiome-based therapies hold great promise for treating various diseases, but the efficient delivery of live bacteria to the colon remains a challenge. Furthermore, current oral formulations, such as lyophilized bacterial capsules or tablets, are produced using processes that can decrease bacterial viability. Consequently, high dosages are required to achieve efficacy. Herein, we report the design of pressure-sensitive colonic capsules for the encapsulation and delivery of aqueous suspensions of live bacteria. The capsules consisted of 2 functional thin-films (hydrophobic and enteric) of ethyl cellulose and Eudragit S100 dip-coated onto hydroxypropyl methylcellulose molds. The capsules could be loaded with aqueous media and provide protection against acidic fluids and, to some extent, oxygen diffusion, suggesting their potential suitability for delivering anaerobic bacterial strains.
Disintegration and mechanical studies indicated that the capsules could withstand transit through the stomach and upper/proximal small intestinal segments and rupture in the ileum/colon. In vitro studies showed that bacterial cells (anaerobic and aerobic commensals) remained highly viable (74–98%) after encapsulation and exposure to the simulated GI tract conditions. In vivo studies with a beagle dog model revealed that 67% of the capsules opened after 3.5 h, indicating content release in the distal gastrointestinal tract. These data demonstrate that live aqueous bacterial suspensions comprised of both aerobic and anaerobic commensals can be encapsulated and in the future might be efficiently delivered to the distal gastrointestinal tract, suggesting the practical applications of these capsules in microbiome-based therapies.
Download the full article as PDF here pH-dependent pressure-sensitive colonic capsules for the delivery of aqueous bacterial suspensions
or read it here
Materials
Ethyl cellulose (EC) (300 cps with a 48% ethoxy substitution), sodium chloride (NaCl), indigo carmine, sodium thiosulfate (Na2S2O3), Evans blue, potassium phosphate monobasic (KH2PO4), sodium hydroxide (NaOH) and bile acids (sodium cholate and sodium deoxycholate) were purchased from Sigma–Aldrich. Ethyl acetate, ethanol and isopropyl alcohol (IPA) were obtained from Acros Organics. Butyl benzoate was purchased from Fluka. Eudragit S100 was obtained from Evonik. Lecithin (from soybean, 90 wt%) was obtained from PanReac Appli Chem. Hydrochloric acid (HCl) was provided by VWR Chemicals. HPMC commercial capsules (size 0) were purchased from Interdelta Switzerland. The Sylgrad™ 184 Silicone Elastomer kit (containing elastomer base and curing agent) was purchased from DOW Europe. Barium sulfate (BaSO4) suspension (Micropaque) was purchased from Guerbet Pharmaceuticals. Carbon steel blades were purchased from Swann-Morton. A sealable cell (117.104-QS), quartz glass (Suprasil, 10 mm Light PathAll) and silicone rubber (septa) were obtained from Altmann Analytik GmbH & Co. KG. LIVE/DEAD™ BacLight™ Bacterial Viability Kit, for microscopy & quantitative assays was purchased from Sigma–Aldrich. Brain heart infusion (BHI) as well as Man–Rogosa–Sharpe (MRS) powder were obtained from Millipore. Symprove™ bacterial suspension containing four bacterial strains: Lactobacillus acidophilus NCIMB 30175, Lactobacillus plantarum NCIMB 30173, Lactobacillus rhamnosus NCIMB 30174 and Enterococcus faecium NCIMB 30176 was ordered directly from the manufacturer (Symprove Ltd., London, England). Bacteroides thetaiotaomicron was obtained from Prof. Slack lab. Hemin and cysteine were obtained from Sigma-Aldrich. Stainless-steel metallic pins for coating were custom designed by the ETH physics workshop (Fig. S3 and Table S3). Chemicals were used as received.
Coating and preparation of the composite capsules
Composite capsules were made of a hydrophobic coating (HC-1 and HC-2) and an enteric coating (EnC-1 and EnC-2). Composite capsules, denoted as HC-1 EnC-1 and HC-2 EnC-2 capsules, were created. Briefly, EC (400 or 600 mg) were dissolved in 10 mL of ethyl acetate overnight under vigorous stirring (1500 rpm) to obtain viscous solutions with 4.5 or 6.6 wt% EC. One layer of 6.6% or 2 layers of 4.5 wt% EC constituted the hydrophobic coating of HC-1 EnC-1 and HC-2 EnC-2, respectively. For the coating step, 3 mL of each solution were transferred into a 4-mL glass flask. Commercially available size 0 HPMC capsules were separated into caps and bodies and then dip-coated either in 4.5 or 6.6 wt% EC solution to obtain the first layer. Coated capsule parts were placed on the designed stainless-steel metallic pins and left to dry at room temperature (RT) for 1 h. Then, the second layer of EC 4.5 wt% was deposited and left to dry for another 30 min at RT. The metallic pins were then transferred to an incubator (37% relative humidity, 27 °C) for further overnight drying. Enteric coatings 1 (EnC-1) and 2 (EnC-2) were prepared as follows: 1 g of Eudragit S100 was vortexed with 0.3 mL or 3 mL of butyl benzoate in a 20-mL glass vial, respectively. Then, the mixture was dissolved overnight in 10 mL (isopropyl alcohol: ethanol) (1:1 v/v). Two layers of EnC-1 or EnC-2 were deposited on either HC-1 or HC-2, respectively, following the same procedure as previously described. The drying procedure was also the same. The final products, HC-1 EnC-1 and HC-2 EnC-2, were stored in an incubator (37% relative humidity, 27 °C) until further use. Body and cap parts were joined and locked like conventional capsules.
Fatma Abdi, Marina Green Buzhor, Nadia Zellweger, Zhi-Luo, Jean-Christophe Leroux, pH-dependent pressure-sensitive colonic capsules for the delivery of aqueous bacterial suspensions, Journal of Controlled Release,
Volume 365, 2024, Pages 688-702, ISSN 0168-3659, https://doi.org/10.1016/j.jconrel.2023.11.048.
Read more on “Disintegrants – Pharmaceutical Excipients” here:


