Structural modifications for the conversion of proteins and peptides into stable dried powder formulations: A review
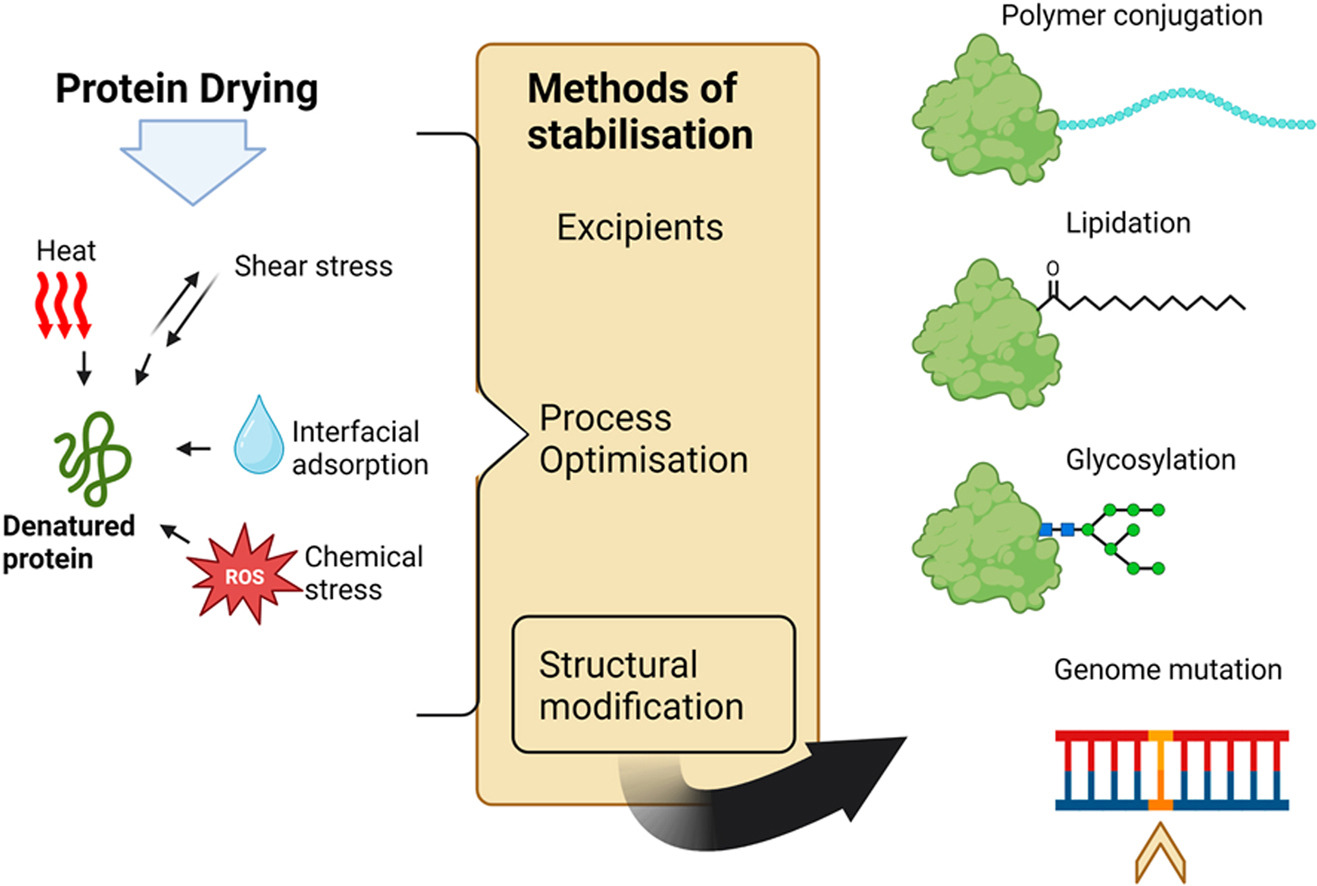
The drying of biomolecules into powdered formulations has become the main form of long-term product stabilisation, allowing for the delivery of safe and efficient medicines. Stability of proteins and peptides during the drying process is paramount for product quality. Drying macromolecules with an appropriate excipient is often sufficient for product stabilisation, however there are limitations imposed on excipient use, particularly in the production of high-value biopharmaceuticals. Innovative approaches for the enhancement of protein stability during dehydration need to be further explored. In this review, we provide a brief discussion of the available drying methods and current stabilisation techniques available for proteins and peptides and review the current impact and limitations of excipient use.
Highlights
- Both established and novel biomolecule drying techniques are discussed in light of their effects on protein and peptide stability.
- Current stabilisation methods for maintaining biomolecule structural integrity during drying are reviewed and their effect on drying applications are recognised.
- Protein engineering, artificial conjugation and post-translational modifications are evaluated in their ability to protect protein integrity during thermal, interfacial and chemical stresses, amongst others.
Methods to induce beneficial protein modifications for drying applications are discussed.
Alongside, we take a detailed look at the impact of post-translational modifications (PTMs) and structural mutations in drying stability of biomolecules. The structural modifications mentioned in this paper are discussed in light of published work on their impact on protein and peptide stability during commonly experienced stresses, particularly those which relate to drying processes, such as chemical, thermal and freeze-thaw. The aim of this review is to direct a research focus towards upstream modifications of proteins and peptides as a viable stabilisation approach during harsh drying processes.
Download the full article as PDF here Structural modifications for the conversion of proteins and peptides into stable dried powder formulations: A review
or read it here
Excpients mentioned in the study: Tween 80, Tween 20, Trehalose, mannitol
Wiktoria Brytan, Luis Padrela, Structural modifications for the conversion of proteins and peptides into stable dried powder formulations: A review, Journal of Drug Delivery Science and Technology, 2023, 104992, ISSN 1773-2247, https://doi.org/10.1016/j.jddst.2023.104992.
See the webinar:
“Enhanced Stability and Bioavailability of Poorly Soluble APIs“,
18 October 2023:
Get more information & register here for free:


