Quality by design aided self-nano emulsifying drug delivery systems development for the oral delivery of Benidipine: Improvement of biopharmaceutical performance
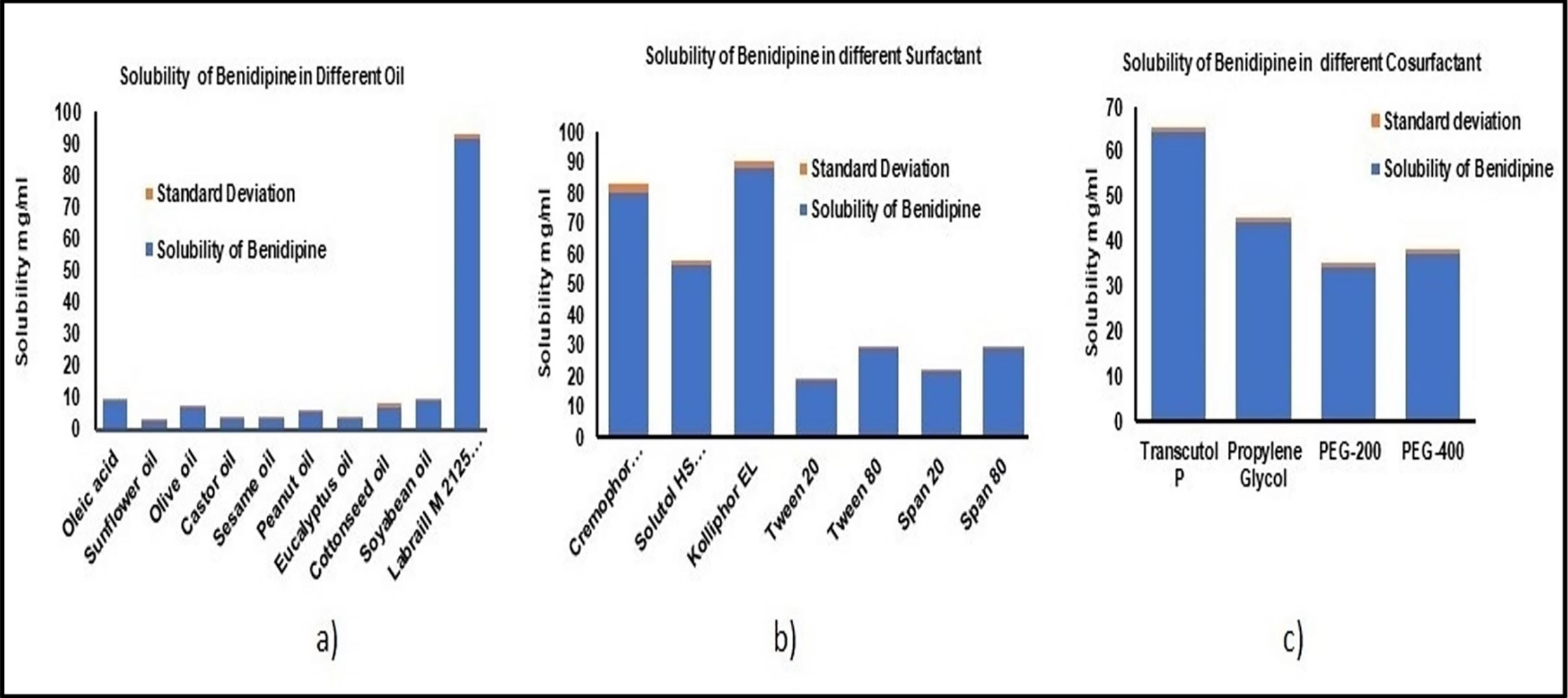
The primary objective of the research effort is to establish efficient solid self-nanoemulsifying drug delivery systems (S-SNEDDS) for benidipine (BD) through the systematic application of a quality-by-design (QbD)-based paradigm. Utilizing Labrafil M 2125 CS, Kolliphor EL, and Transcutol P, the BD-S-SNEDDS were created. The central composite design was adopted to optimize numerous components. Zeta potential, drug concentration, resistance to dilution, pH, refractive index, viscosity, thermodynamic stability, and cloud point were further investigated in the most efficient formulation, BD14, which had a globule size of 156.20 ± 2.40 nm, PDI of 0.25, zeta potential of −17.36 ± 0.18 mV, self-emulsification time of 65.21 ± 1.95 s, % transmittance of 99.80 ± 0.70%, and drug release of 92.65 ± 1.70% at 15 min.
S-SNEDDS were formulated using the adsorption process and investigated via Fourier transform infrared spectroscopy, Differential scanning calorimeter, Scanning electron microscopy, and powder X-ray diffraction. Optimized S-SNEDDS batch BD14 dramatically decreased blood pressure in rats in contrast to the pure drug and the commercial product, according to a pharmacodynamics investigation. Accelerated stability tests validated the product’s stability. Therefore, the development of oral S-SNEDDS of BD may be advantageous for raising BD’s water solubility and expanding their releasing capabilities, thereby boosting oral absorption.
Download the full article as PDF here Quality by design aided self-nano emulsifying drug delivery systems development for the oral delivery of Benidipine
or read it here
Materials
BD was acquired as a free sample from Nikishan Pharmaceutical (Ankleshwar, Gujarat, India). Cremophor® RH 40 and Solutol® HS were provided by BASF (Mumbai, India). A free sample of Labrafil® M 2125 CS and Transcutol P was offered by Gattefosse (Mumbai, India). Tween® 20, Tween® 80, Span® 20, Span® 80, polyethylene glycol 400 (PEG 400), polyethylene glycol 200 (PEG 200), propylene glycol (PG), oleic acid, sunflower oil, sesame oil, olive oil, castor oil, peanut oil, eucalyptus oil, cottonseed oil and sodium lauryl sulfate (SLS) were obtained from SD Fine Chemicals (Mumbai, India). Water that had been repeatedly distilled was the solvent for the entire experiment. All of the additional chemicals employed in this investigation were of the analytical kind. Torrent Research Center (Ahmedabad, India) voluntarily provided empty hard gelatin capsules. Fuji Chemical Industries (Burlington, NJ, USA) provided Neusilin US2, Vardhman Healthcare (Ahmedabad, India) offered Aerosil 200, and Evonik Industries (Mumbai, India) provided Aeroperl 300.
Quality by design aided self-nano emulsifying drug delivery systems development for the oral delivery of Benidipine: Improvement of biopharmaceutical performance, Sheetal S. Buddhadev,Kevinkumar C. Garala,Saisivam S,Mohamed Rahamathulla,Mohammed Muqtader Ahmed,Syeda Ayesha Farhana &Ismail Pasha, Article: 2288801 | Received 06 Sep 2023, Accepted 12 Nov 2023, Published online: 11 Dec 2023, https://doi.org/10.1080/10717544.2023.2288801
Watch our free webinars to “Quality by Design” here:


