Development of rapidly soluble mebendazole nanosuspension for colorectal cancer
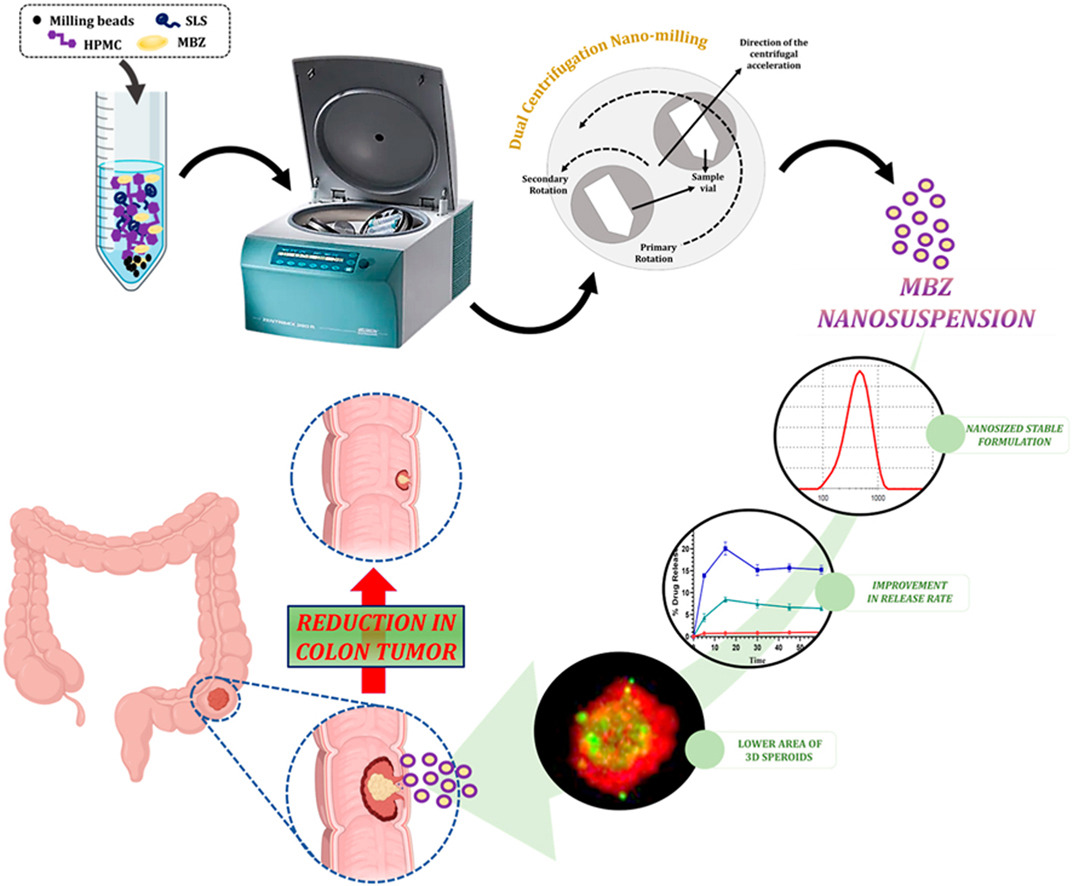
Mebendazole (MBZ) has been proven as a repurposing molecule against colorectal cancer. Unfortunately, its clinical application is constrained by its extremely poor solubility and bioavailability. The aim of the current work was to augment the dissolution rate at colonic pH and the anticancer efficacy by formulating MBZ nanosuspension (NS). A robust MBZ NS was developed using a combination of Sodium Lauryl Sulphate (SLS) and HydroxyPropylMethyl-Cellulose E5 (HPMC) as stabilizers through the dual centrifugation method.
The prepared MBZ NS exhibited the smallest particle size (362.6 ± 12.3 nm) with unimodal particle size distribution and excellent short-term stability. DSC and FT-IR studies confirmed the crystallinity and the absence of interactions with the stabilizers used. The developed MBZ NS demonstrated ∼20 folds higher dissolution than MBZ alone in colonic pH. Cell experiments showed that HPMC E5 and SLS were safe on HT-29 and HT-116 cell lines. In addition, IC50 of MBZ-NS was found to be 1.028 ± 0.030 μM and 0.884 ± 0.050 μM in HT-29 and HT-116 cell lines, respectively.
Furthermore, 1.5 folds and 1.8 folds reduction in 3D tumor spheroids after treatment with MBZ-NS was observed compared to the control and MBZ physical mixture. From the In Vitro Live/Dead Cell Assay higher amount of red fluorescence in treatment groups confirmed a large number of dead cells compared to the control. In a nutshell, the produced MBZ NS could be employed as a promising formulation to achieve a higher dissolution rate and anticancer efficacy against colorectal cancer of repurposing drug MBZ.
Introduction
Colorectal cancer (CC) was infrequently diagnosed but has now become the fourth leading cause of cancer related deaths worldwide, and the second most prevalent cause of cancer death in both men and women (approximately 9,00,000 deaths annually) [1]. Until now, CC primarily affected individuals over the age of 50; however, over the past decade, there has been a significant rise in cases among younger people. Due to bad lifestyle behaviors among youngsters, it is likely to diagnose under the age of 40 [2].
Understanding the pathophysiological state has expanded the range of available treatments for early and late stages, resulting in customized therapy regimens. Current treatments, such as surgical local excision, chemotherapy, immunotherapy, targeted therapy, radiotherapy, and endoscopy, have become the preferred approaches for managing individuals with CC [3]. Chemotherapy and radiation, however, have certain drawbacks and come with several risks, including tissue toxicity that can affect both cancerous and healthy cells [4]. Additionally, targeted treatments have the potential to influence immune responses, suggesting that they may work well in conjunction with immunotherapy to enhance clinical results [5].
The efficacy of novel molecular discovery is constrained by the high cost involved in drug development to find a robust candidate and bring it through all the studies required for FDA approval. Drug repurposing, which involves finding new uses for already-approved drugs, is gaining attention as a way to speed up the approval process, reduce the burden on the economy, and reduce the risk of failure owing to side effects. The preferred choice for drug repurposing is a molecule with low cost, well-established safety profile, pre-clinical efficacy data, and pharmacokinetics that enable therapeutic concentrations to be reached at disease site [6].
Mebendazole (MBZ), a broad-spectrum anthelmintic benzimidazole, has demonstrated preclinical anti-cancer activity in a wide range of cancers including CC, lung, brain, and many more [7,8]. MBZ blocks tumor-promoting factors such as vascular endothelial growth factor receptor 2 (VEGFR2), tubulin polymerization, matrix proteinases, pro-survival pathways, and protein transporters for multiple drug resistance [9]. MBZ has been repurposed for the treatment of CC in case reports and clinical findings for this treatment are now being conducted [8,10]. MBZ (Fig. 1) is a biopharmaceutical classification system (BCS) II drug with a very high daily dose (≈100–500 mg). It shows pH-dependent poor water solubility and bioavailability (17–20 %) due to the inadequate absorption and extensive first-pass effect [11,12].
Therefore, MBZ must be reformulated using the benefits of nanotechnology that can deliver drugs specifically at the desired site of action, prevent first-pass metabolism and improve drug effectiveness [13].
Nanosuspension (NS), also known as nanocrystals, is a unique system composed of drugs and stabilizers having nano-range particle size, generally ranging from 10 to 1000 nm. The technique works effectively for the molecules (MBZ) with physicochemical properties like high crystallinity, high melting point (288.5 °C), poor aqueous and non-aqueous solubility, and high molecular weight (295.3 Da) [14]. The technology might be useful for brick dust compounds with the advantages of improved efficiency in terms of saturated solubility, fast dissolution, absence of Ostwald ripening, and large-scale versatility for the production of BCS class II and IV molecules.
Considering this background, the current work was designed to formulate nanosuspension of MBZ using a recently developed dual centrifugation (DC) nano-milling machine. Our study’s intended approach for oral MBZ delivery encompasses the use of an enteric-coated capsule, which strategically safeguards MBZ from the acidic stomach environment, facilitating its targeted release in the colon. Also plays a pivotal role in solubility and bioavailability enhancement of MBZ, with a specific focus on its application against colorectal cancer. Our hypothesis proposed that the oral administration of MBZ nanosuspension would lead to a significant localized concentration in the colon, effectively targeting regional tumor cells. Physicochemical evaluations such as mean particle size, Zeta potential, short-term stability, drug release, and material characteristics were optimized systematically. Cell culture studies against colon cancer cell lines (HT-29 and HT-116) were performed to evaluate the efficacy of MBZ NS.
Materials
MBZ was procured from AK Scientific (Union City, CA, USA). Hydroxypropyl methyl cellulose E5 (HPMC) was purchased from Dow Chemical Company (Midland, MI, USA). Kolliphor SLS (SLS), Kollidon® VA 64 (VA 64), and d-alpha tocopheryl polyethylene glycol 1000 succinate (Kolliphor® TPGS; TPGS) were generously provided by BASF Corporation (Tarrytown, NY, USA). Tween 80 was obtained from Croda (Edison, NJ, USA). Dimethyl sulfoxide (DMSO) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
Read more
Rajeshri D. Patel, Akanksha S. Patel, Henis J. Patel, Sruthi Sarvepalli, Ketan Patel, Development of rapidly soluble mebendazole nanosuspension for colorectal cancer, Journal of Drug Delivery Science and Technology, Volume 91, 2024, 105276, ISSN 1773-2247,
https://doi.org/10.1016/j.jddst.2023.105276.

