Telmisartan Tablets Repackaged into Dose Administration Aids: Physicochemical Stability under Tropical Conditions
Abstract
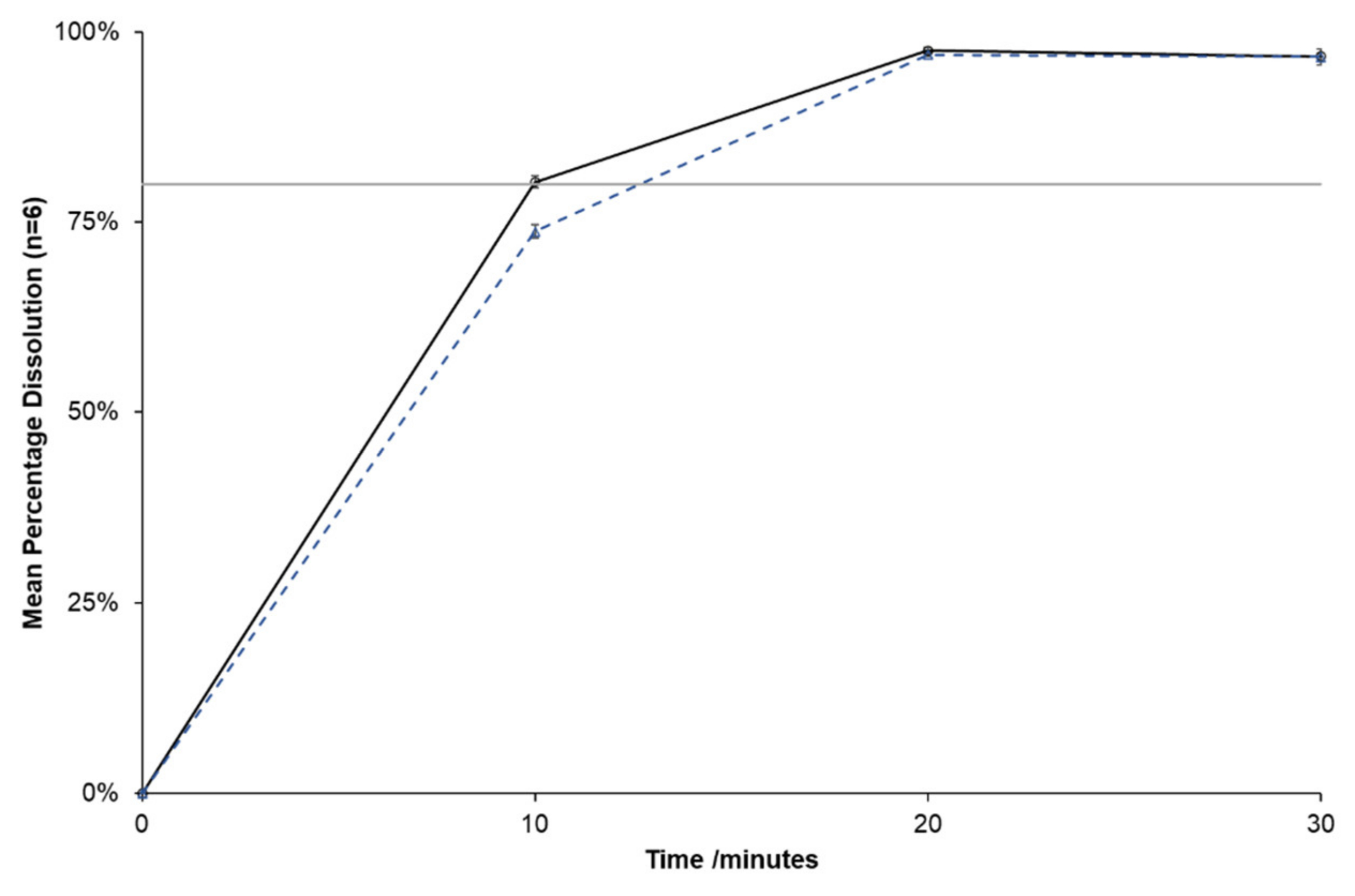
Dose administration aids (DAAs) are commonly used to assist patients with chronic disease to manage multiple medications and thus improve adherence. Several brands of telmisartan, commonly prescribed for hypertension, are available in Australia. Manufacturer’s storage advice is to leave tablets in the blister strip until administered to patients. This study aimed to investigate the stability of telmisartan tablets when repackaged and stored in DAAs, to identify a brand, which is sufficiently stable to be repackaged. All available brands of telmisartan tablets in Australia, which contain different excipients, were repackaged into DAAs and stored at 30 °C, 75% RH for 28 days before screening, using visual inspection and physical testing. A candidate brand was then selected for physicochemical and photostability testing using pharmacopoeial methods. Repackaged Mizart® tablets were shown to be sufficiently stable, when repackaged and stored under tropical conditions (30 °C, 75% RH) for 28 days. Several of the other brands were deemed inappropriate for repackaging, due to physical instability, highlighting the importance of considering not only the drug, but also excipients to ensure the stability of repackaged medicines. Although the repackaging of telmisartan tablets is not advised, this study provides evidence to support the Mizart® brand as an option for pharmacists to recommend for repackaging.
1. Introduction
| Tablet Brand | Excipient |
|---|---|
| Micardis® | Sorbitol |
| DRLA® | Mannitol, Polysorbate 80 |
| APO® | Mannitol, Sodium stearyl fumarate |
| Mizart® | Mannitol, Sodium stearyl fumarate |
| GH® | Mannitol, Sodium stearyl fumarate |
| Teltartan® | Mannitol, Sodium stearyl fumarate |
| Sandoz® | Lactose, Ludipress |
| Pharmacor® | Lactose, Crospovidone, Colloidal anhydrous silica |
Download the full study as PDF here Telmisartan Tablets Repackaged into Dose Administration Aids: Physicochemical Stability under Tropical Conditions
or read it here
Ma, A.P.; Robertson, S.G.; Glass, B.D. Telmisartan Tablets Repackaged into Dose Administration Aids: Physicochemical Stability under Tropical Conditions. Pharmaceutics 2022, 14, 1667. https://doi.org/10.3390/pharmaceutics14081667

