A review on natural biopolymers in external drug delivery systems for wound healing and atopic dermatitis
Abstract
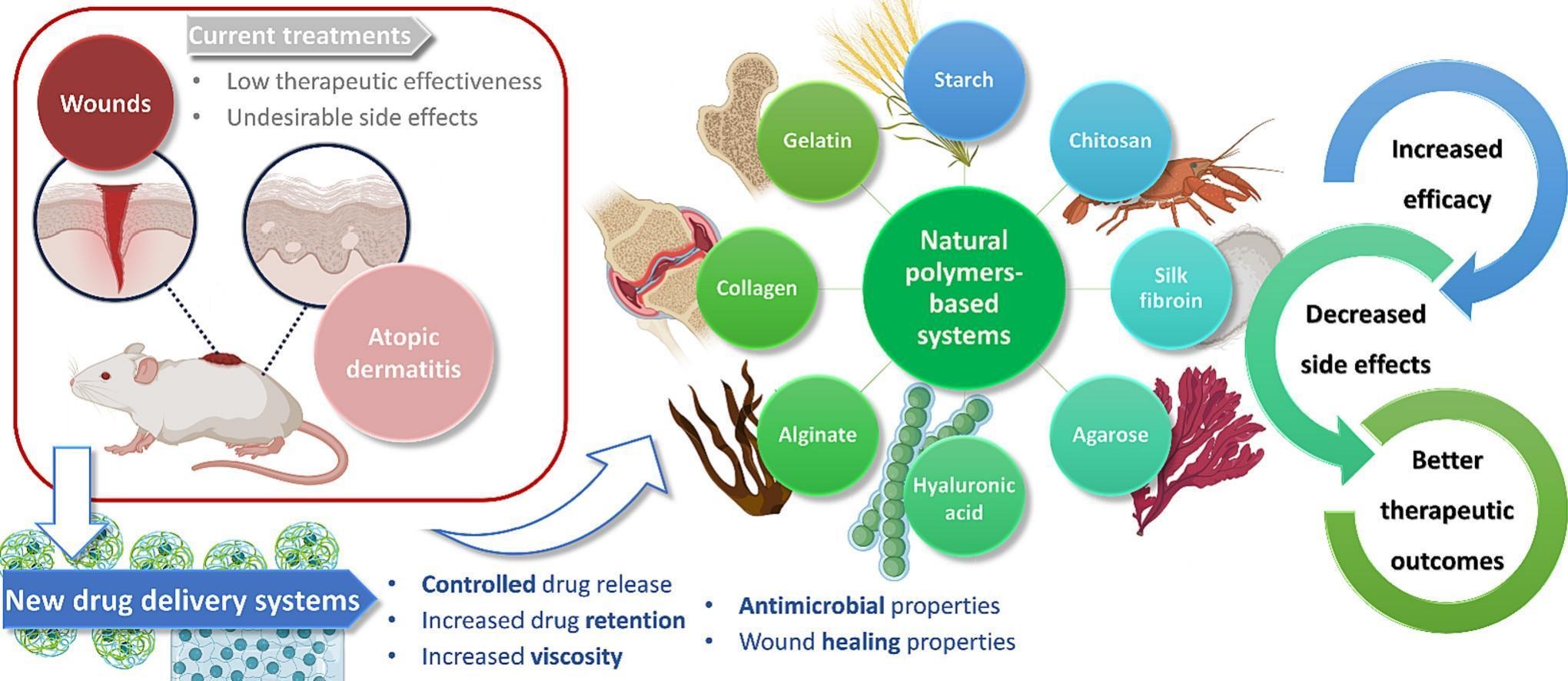
Despite the advantages of topical administration in the treatment of skin diseases, current marketed preparations face the challenge of the skin’s barrier effect, leading to low therapeutic effectiveness and undesirable side effects. Hence, in recent years the management of skin wounds, the main morbidity-causing complication in hospital environments, and atopic dermatitis, the most common inflammatory skin disease, has become a great concern. Fortunately, new, more effective, and safer treatments are already under development, with chitosan, starch, silk fibroin, agarose, hyaluronic acid, alginate, collagen, and gelatin having been used for the development of nanoparticles, liposomes, niosomes and/or hydrogels to improve the delivery of several molecules for the treatment of these diseases. Biocompatibility, biodegradability, increased viscosity, controlled drug delivery, increased drug retention in the epidermis, and overall mitigation of adverse effects, contribute to an effective treatment, additionally providing intrinsic antimicrobial and wound healing properties. In this review, some of the most recent success cases of biopolymer-based drug delivery systems as part of nanocarriers, semi-solid hydrogel matrices, or both (hybrid systems), for the management of skin wounds and atopic dermatitis, are critically discussed, including composition and in vitro, ex vivo and in vivo characterization, showing the promise of these external drug delivery systems.
Introduction
The treatment of skin diseases through topical drug administration offers significant advantages compared to systemic routes. These advantages include targeted drug delivery, minimized systemic drug exposure (and, consequently, fewer systemic side-effects), and avoidance of hepatic first-pass effect [[1], [2], [3]]. Topical formulations are also easy and painless to administer (non-invasiveness), ensuring good patient compliance. They can be tailored to have controlled drug release, leading to therapeutic effects that last for a longer period of time [[3], [4], [5]]. Nevertheless, marketed preparations face considerable challenges, since the skin’s barrier effect makes it difficult for drugs to permeate it, with existing therapies having lack of effectiveness, need of repeated dosing, and local as well as systemic side effects [[6], [7], [8]]. Hence, the development of new, more effective, and safer treatments is greatly needed.
Among the various diseases and injuries that can affect the skin, atopic dermatitis (AD) and wounds have become a growing concern, not only for patients but also for healthcare professionals [9,10]. AD is the most common inflammatory cutaneous disease, being characterized by a disruption of the skin barrier that leads to increased transepidermal water loss (TEWL) and inflammation [11,12]. In addition, the impairment of the skin barrier may trigger secondary infections, namely infections by Staphylococcus aureus [13]. Therefore, the treatment of AD focuses on repairing the skin barrier, reducing inflammation processes, and bacterial infections. Additionally, skin hydration plays an important role in AD, as it improves dryness, pruritus and helps with the restoration of the compromised stratum corneum [14]. Corticosteroids are the mainstay of AD treatment, traditionally applied through creams and ointments. However, in these conventional formulations, corticosteroids have several topical and systemic side effects that limit their applicability in chronic treatment regimens, namely allergic contact dermatitis, TEWL increase, telangiectasia, reduction of keratinocytes after 3 to 4 weeks, adrenal suppression, among others [[15], [16], [17], [18], [19]]. Fortunately, corticosteroid-loaded nanocarriers have been developed and incorporated into creams, ointments, and chitosan gels, which have shown increased viscosity for suitable topical administration, controlled drug delivery, increased drug retention in the epidermis (site of the inflammation process), and mitigation of the adverse effects of corticosteroids [9,17,18].
Conversely, the incidence of skin wound infections and chronic skin wounds has steadily increased in the past few years. While acute wounds usually heal spontaneously, chronic wounds exhibit a high pH value, necrotic tissue, and a high number of metalloproteases, impeding the physiological process of wound healing [20]. The most common bacteria associated with wound infection are Staphylococcus aureus and Pseudomonas aeruginosa. Fighting wound bacterial infection is a great challenge because they have the ability to form biofilms and present increasing antibiotic resistance, which delays the healing process, increases morbidity, and also makes health costs rise [21,22]. Several antibacterial agents have been used to manage wounds, mainly antiseptics, antibiotics, and metallic nanoparticles (NP). Traditionally, antiseptics and antibiotics are administered topically in the form of creams, ointments, and gels. In these conventional formulations, there is a higher chance of these drugs not reaching the adequate concentration at the site of infection, due to their degradation in the wound environment, difficulty in penetrating biofilms, short application period and rapid clearance. The resulting inadequate dosing accuracy can potentiate antiseptics toxic effects on human cells, and lead to the development of contact dermatitis and bacterial resistance caused by antibiotics [20]. Furthermore, conventional semi-solid bases are poorly suited for wound exudate absorption, and metallic NPs have also shown cytotoxic effects [23]. On the other hand, traditional dry dressings such as gauze, cotton wool, and synthetic bandages do not provide the moist environment necessary for wound healing. Also, polyurethane film dressings do not absorb exudates enough, silicone or polyurethane foam requires a secondary dressing to adhere to the skin, and hydrocolloids are cytotoxic, not providing the ideal environment for accelerated wound healing [20]. To tackle these issues, a large panoply of new drug delivery systems (DDS) has emerged, in which hydrogels based on natural polymers have had a relevant role, attending to their favorable features, namely provision of a moist environment, absorption of wound exudates, controlled release of active agents and nanocarriers, among others [[24], [25], [26], [27], [28]].
Additionally, natural polymers have several other relevant features that make them suitable for drug delivery in general, such as their biocompatibility and biodegradability, low cost and easy access, and intrinsic bioactivity, with antibacterial and wound healing effects being quite useful when meant for topical application in the treatment of AD and wound healing [[29], [30], [31], [32]]. These polymers can be part of the composition of the nanocarriers themselves, form semi-solid hydrogel matrices, or both, making the so called hybrid systems, with several hybrid systems for the management of AD having already emerged in the scientific literature, namely chitosan NPs, starch NPs and liposomes incorporated into creams, Carbopol® gels or chitosan gels, to improve the delivery of corticosteroids, hydroxytyrosol (HT) or phytoceramides [9,[15], [16], [17], [18],[33], [34], [35], [36]]. In parallel, hybrid systems for the management of skin wounds have also emerged, namely liposomes, silver nanoparticles (AgNPs), zinc oxide nanoparticles (ZnO NPs), silica NPs, halloysite clay nanotubes (HNTs) and niosomes, which have been incorporated into chitosan, silk fibroin, agarose, hyaluronic acid (HA), alginate, collagen or gelatin hydrogels, to improve the delivery of chlorhexidine (CHX), Ag+, Aloe vera, mupirocin, gentamicin, rifamycin, vancomycin, ciprofloxacin and polymyxin B sulfate [10,21,22,24,[37], [38], [39], [40], [41], [42], [43], [44]].
In this review, some of the most recent success cases of natural polymer DDSs development for the management of AD and skin wounds will be critically discussed (summary in Fig. 1), including their composition and in vitro, ex vivo and in vivo characterization, showing the promise of these types of systems for the future of topical treatments.
Read more here
Patrícia C. Pires, Fouad Damiri, Ehsan Nazarzadeh Zare, Anwarul Hasan, Rasoul Esmaeely Neisiany, Francisco Veiga, Pooyan Makvandi, Ana Cláudia Paiva-Santos, A review on natural biopolymers in external drug delivery systems for wound healing and atopic dermatitis, International Journal of Biological Macromolecules, Volume 263, Part 1, 2024, 130296, ISSN 0141-8130, https://doi.org/10.1016/j.ijbiomac.2024.130296.
Read also our introduction article on Chitosan here:


