Correlating mechanical and rheological filament properties to processability and quality of 3D printed tablets using multiple linear regression
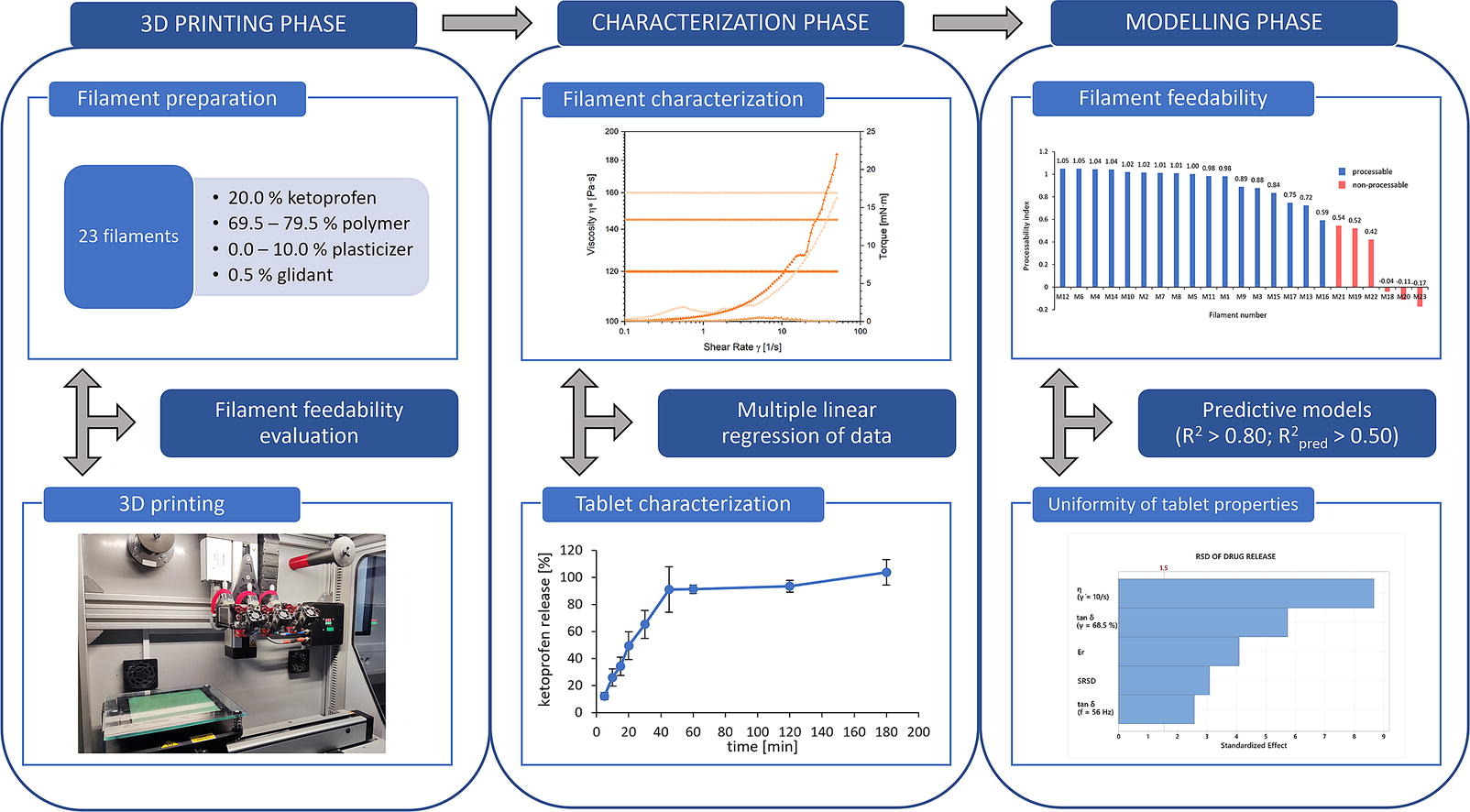
Filament formulation for FDM is a challenging and time-consuming process. Several pharmaceutical polymers are not feedable on their own. Due to inadequate filament formulation, 3D printed tablets can also exhibit poor uniformity of tablet attributes. To better understand filament formulation process, 23 filaments were prepared with the polymer mixing approach. To yield processable filaments, brittle and pliable polymers were combined. A 20 % addition of a pliable polymer to a brittle one resulted in filament processability and vice versa. Predictive statistical models for filament processability and uniformity of tablet attributes were established based on the mechanical and rheological properties of filaments. 15 input variables were correlated to 9 responses, which represent filament processability and tablet properties, by using multiple linear regression approach. Filament stiffness, assessed by indentation, and its square term were the only variables that determined the filament’s feedability. However, the resulting model is equipment-specific since different feeding mechanism exert different forces on the filaments. Additional models with good predictive power (R2pred > 0.50) were established for tablet width uniformity, drug release uniformity, tablet disintegration time uniformity and occurrence of disintegration, which are equipment-independent outputs. Therefore, the obtained model outcomes could be used in other research endeavours.
1. Introduction
Since the expiration of key patents in additive manufacturing and approval of the first 3D printed medication, 3D printing has experienced considerable activity in the medical field. The technology is attractive to say the least, as it allows complete control over the design of the printed object. Growing awareness of the benefits of widespread use of personalized medicine, supported by the achievements of pharmacogenomics, emerged at virtually the same time, linking both personalized medicine and manufacturing technology into a potential platform for drug manufacturing of the future. Tailored dimensions and dosage strength for each individual patient would greatly improve the quality of life, boost treatment effectiveness, reduce drug adverse effects and increase patient compliance (Siamidi et al., 2020, Trenfield et al., 2018). Various implementations of this rapid 3D prototyping technology have been developed, such as fused deposition modelling (FDM), selective laser sintering, stereolithography, binder jetting, semi-solid extrusion and many more (Jamróz et al., 2018).
FDM is especially enticing due to low cost, wide variety of 3D printer manufacturers and relatively short production times (Jamróz et al., 2018, Lamichhane et al., 2019). Many researchers have proved the viability of FDM for production of 3D printed solid dosage forms (Tan et al., 2018). Furthermore, connecting hot-melt extrusion (HME) with the 3D printing process provides a capable machinery for personalized medicine production in a two-step process. HME is first utilized to turn a polymer-based powder mixture into the filament, a feedstock for 3D printing. The filament is subsequently loaded into the FDM 3D printer via a filament spool. Upon passing the heated nozzle, the polymer changes from a solid glassy to a viscoelastic rubbery state. The polymer’s viscosity is reduced, and the significantly softened filament is deposited onto the print bed in several layers. The material then cools down, as the individual layers merge together into a solid structure. 3D printer deposits the filament based on the computer-aided design (CAD) (Jamróz et al., 2018).
Most pharmaceutical thermoplastic polymers show a lot of promise for hot-melt extrusion and filament formation due to high thermal stability, good solubilization capacity, adequate viscosity at printing temperature and biocompatibility. Polyvinyl alcohol (PVA), polyvinyl pyrrolidone (PVP), polyvinyl alcohol-polyethylene glycol graft copolymer (Kollicoat®IR), cellulose derivatives (HPC, HPMC, EC, HPMCAS), polymethacrylate-based copolymers etc. have all been investigated as potential API carriers (Azad et al., 2020, Melocchi et al., 2016, Palekar et al., 2019, Pietrzak et al., 2015, Zhang et al., 2017). However not many are printable on their own, mostly due to excessive brittleness or pliability. In the pharmaceutical industry, it is desirable that HME extrudates exhibit brittle behavior to allow for postprocessing, such as comminution, pellet or tablet manufacturing. On the other hand, filaments for 3D printing require a compromise between pliability, hardness and stiffness to pass the nozzle without excessive damage (Aho et al., 2019, Nasereddin et al., 2018, Palekar et al., 2019).
The filament feeder aggressively handles the filament during the feeding process. The drive and pinch wheels squeeze the feedstock material and send it into the printing head liquefier for material deposition at high shear rates. During the feeding process, the filament can become damaged with small indentations (Go et al., 2017). This can result in filament breakage within the feeder. In addition, soft and pliable filaments can buckle before or within the printhead. The filament gets stuck when entering the liquefier due to the lack of column strength along the material (Ilyés et al., 2019, Solanki et al., 2018). Filament buckling can be otherwise somehow managed by establishing a critical ratio between the filament’s elastic modulus and apparent viscosity (Venkataraman et al., 2000). Filament residue within the printhead is also problematic from the cleaning standpoint, as the mechanism needs to be completely disassembled to remove the debris and residuals (Nasereddin et al., 2018). As a result, good mechanical properties of the filament should be designed for effortless processability. Polymer blending and the addition of excipients (non-organic fillers, plasticizers etc.) are a promising solution to reach desired mechanical and rheological properties (Aho et al., 2019, Fuenmayor et al., 2018).
Once the filament melts within the liquefier, it is deposited via a heated nozzle onto the print bed. Accuracy of material deposition defines the uniformity of the printing process which is crucial for the technology adoption. Material flow should be steady without potential air bubbles or nozzle clogging. Melt viscosity, printing temperature and filament thickness variation can all impact the quality of final dosage forms (Aho et al., 2019). Optimal printing temperature can be hard to determine. On one hand it is limited by high melt viscosity, while high temperatures can lead to drug and/or polymer degradation (Aho et al., 2019). Rheological properties of filaments are therefore crucial to assess the material’s performance during material deposition. However, investigation into the uniformity of the 3D printed tablet’s characteristics is largely neglected.
Filament formulation on can be a challenging process. Trial and error approach is wasteful, time-consuming and costly. Currently, there is no definitive filament property profile established, which would serve as a guidance for material selection and printability (Fuenmayor et al., 2018). Development of predictive models is necessary to facilitate the formulation process. In this way, filament composition could be improved not only to assure filament feedability, but also to promote the reproducibility of printed layers merging, thereby improving the uniformity of tablet properties to accommodate the pharmacopeial requirements for tablets.
A few mechanical screening techniques for filament formulation have already been introduced with a varying degree of success. The Zhang-Repka testing methodology features the use of a texture analyzer in a bending and indentation mode to assess brittleness, flexibility and stiffness of the filament specimen. By using the three-point bend test holder, the test is carried out on a short piece of the filament. The top blade either displaces or indents the filament, while force, and distance are measured (Zhang et al., 2019, 2017). Based on the observations, filaments with the following characteristics are processable: breaking distance > 0.61 mm, breaking stress > 635.5 g/mm2 and stiffness > 20758.3 g/mm2 (Zhang et al., 2019). Another screening compression test was developed with a texture analyzer where filament was mounted between two far-end caps. Force is recorded as the caps move towards one another and the measurements are characterized in feedable, tunable and non-feedable groups of filaments (Nasereddin et al., 2018). Xu et al. tested both methods and the stiffness test appeared to be the most accurate screening technique. The stiffness threshold for printability was determined at 80 g/mm2 (Xu et al., 2020). However, availability of various 3D printers and hardware make it hard to generalize a design space across all equipment (Henry et al., 2021). Bowden and direct extrusion FDM 3D printers certainly differentiate in filament requirements for processability, as the feeding process varies (Fuenmayor et al., 2018, Xu et al., 2020). Other mechanical and rheological tests (tensile strength, fracturability, melt flow indexing, complex viscosity etc.) are occasionally performed to explain certain filament characteristics (Fuenmayor et al., 2018, Gültekin et al., 2019, Henry et al., 2021, Samaro et al., 2020, Shi et al., 2021, Solanki et al., 2018, Than and Titapiwatanakun, 2021). However, a systematic approach to identify key filament properties is still lacking. As rheological and mechanical properties of filaments might impact not only the processability, but also the consistency of material deposition, consistency of interlayer merging and uniformity of tablet attributes, several filament properties were measured and correlated in this study. The aim of this article is therefore to identify key properties of filaments which are responsible for processability and the uniformity of the critical quality attributes of tablets.
Lastly, important novelty in in silico computational approaches to predict extrusion and printing temperatures, filament mechanical characteristics, printability and drug release of 3D printed tablets were achieved (Muñiz Castro et al., 2021, Ong et al., 2022). Input data was obtained from in-house measurements and extensive data mining of published literature. Filament composition, physical properties, equipment specifics and tablet design were considered to set predictive models with a high degree of accuracy. The open-access software M3DISEEN can be used in preliminary trials to screen a wide variety of formulations for printing suitability.
2.1. Materials for extrusion
Active pharmaceutical ingredient ketoprofen and all other excipients were obtained internally from Lek pharmaceuticals d.d.. 12 pharmaceutical grade polymers were screened for printing suitability: Soluplus® (polyvinyl caprolactam–polyvinyl acetate–polyethylene glycol graft copolymer), Kollidon® 30 (polyvinylpyrrolidone), Kollidon® VA 64 (vinylpyrrolidone-vinyl acetate copolymer), Klucel™ EF (hydroxypropyl cellulose), Klucel™ LF (hydroxypropyl cellulose), Plasdone™ K-17 (polyvinylpyrrolidone), Plasdone™ K-25 (polyvinylpyrrolidone), Polyox™ WSR N750 (polyethylene oxide), Affinisol™ HPMC HME 15 LV (hydroxypropyl methylcellulose), Parteck® MXP (polyvinyl alcohol), Shin-Etsu AQOAT® AS-LG (hydroxypropyl methylcellulose acetate succinate) and Nisso HPC SSL (hydroxypropyl cellulose). Plasticizers Parteck® M200 (mannitol) or Polyglykol® 3350 P (polyethylene glycol) were introduced into certain physical mixtures to facilitate the extrusion process. To improve flowability of the powder blend, glidant Syloid® 244 FP (fumed silica) was added to all physical mixtures.
Download the full study Pre-proof as PDF here: Correlating mechanical and rheological filament properties to processability and quality of 3D printed tablets using multiple linear regression – Pre-proof
or read it here
Klemen Kreft, Zoran Lavrič, Urška Gradišar Centa, Mohor Mihelčič, Lidija Slemenik Perše, Rok Dreu, Correlating mechanical and rheological filament properties to processability and quality of 3D printed tablets using multiple linear regression, International Journal of Pharmaceutics, 2023, 123719, ISSN 0378-5173,
https://doi.org/10.1016/j.ijpharm.2023.123719.

