Sample Size Requirements of a Pharmaceutical Material Library: A Case in Predicting Direct Compression Tablet Tensile Strength by Latent Variable Modeling
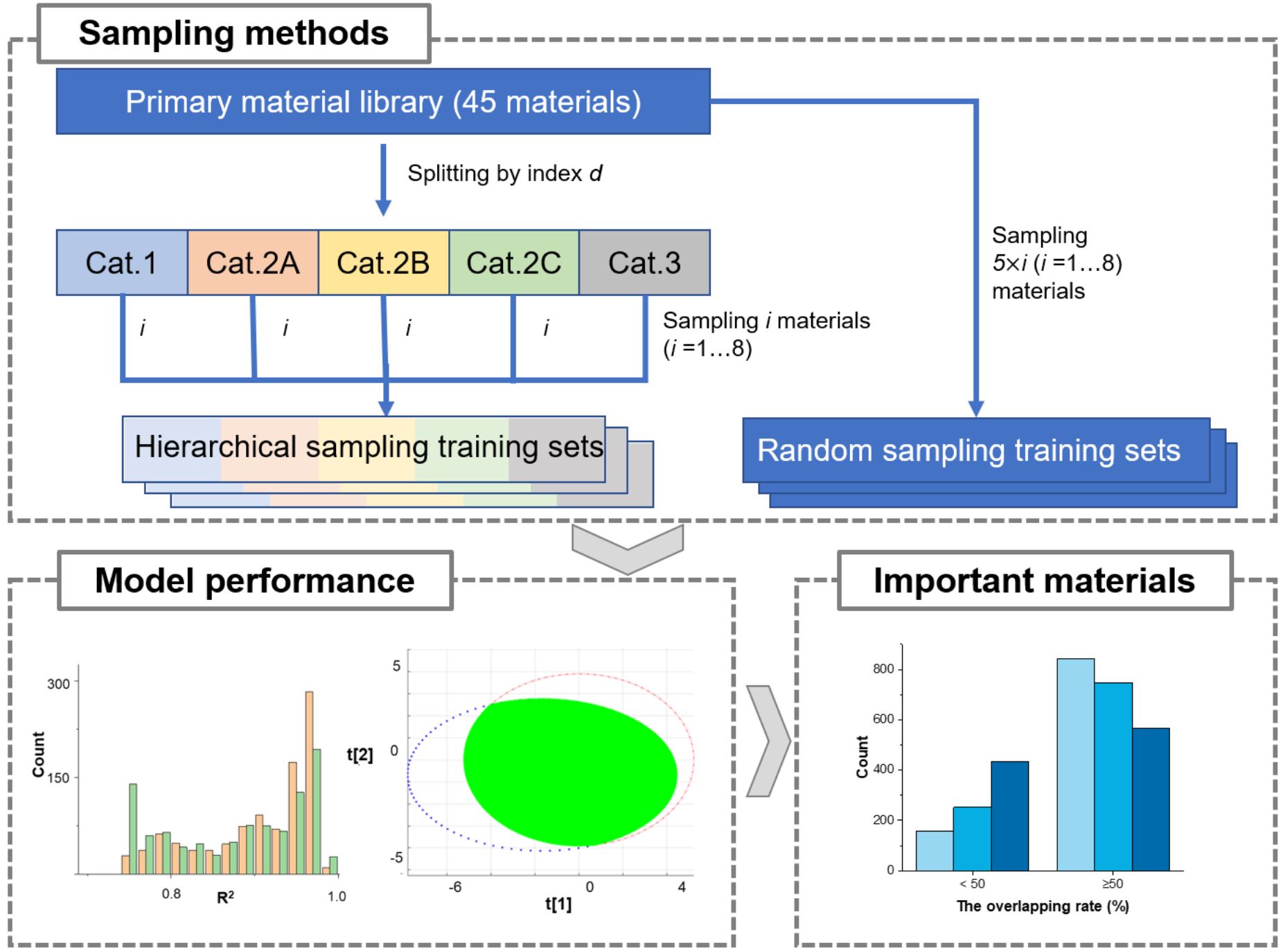
The material library is an emerging, new data-driven approach for developing pharmaceutical process models. How many materials or samples should be involved in a particular application scenario is unclear, and the impact of sample size on process modeling is worth discussing. In this work, the direct compression process was taken as the research object, and the effects of different sample sizes of material libraries on partial least squares (PLS) modeling in the prediction of tablet tensile strength were investigated. A primary material library comprising 45 materials was built. Then, material subsets containing 5 × i (i = 1, 2, 3, …, 8) materials were sampled from the primary material library.
Each subset underwent sampling 1000 times to analyze variations in model fitting performance. Both hierarchical sampling and random sampling were employed and compared, with hierarchical sampling implemented with the help of the tabletability classification index d. For each subset, modeling data were organized, incorporating 18 physical properties and tableting pressure as the independent variables and tablet tensile strength as the dependent variable. A series of chemometric indicators was used to assess model performance and find important materials for model training. It was found that the minimum R2 and RMSE values reached their maximum, and the corresponding values were kept almost unchanged when the sample sizes varied from 20 to 45.
When the sample size was smaller than 15, the hierarchical sampling method was more reliable in avoiding low-quality few-shot PLS models than the random sampling method. Two important materials were identified as useful for building an initial material library. Overall, this work demonstrated that as the number of materials increased, the model’s reliability improved. It also highlighted the potential for effective few-shot modeling on a small material library by controlling its information richness.
Download the full article as PDF here: Sample Size Requirements of a Pharmaceutical Material Library
or read it here
Materials
A total of 45 powdered materials, including 32 pharmaceutical excipients and 13 natural production powders (NPPs), were carefully selected from a homemade database named intelligent TCM (iTCM) [4,37]. Different batches or types of the same material, exhibiting different capacities, were considered in the material library. For instance, seven types of MCC powders, including PH102, PH200NF, Oricel™PH-102 SCG, Oricel™PH302NF, Oricel™PH-112, Oricel™PH302NF, and vivapur® type200, were included. These 45 samples were used as the primary material library and were divided into 5 categories (Cat.1, 2A, 2B, 2C, 3; Cat.1 denotes Category 1, and the same nomenclature applies to the others) by the tabletability index d. Each category included 9 samples. The names, lot numbers, and suppliers for all materials are described in Table S2 in the Supplementary Materials.
Table S2. The information for 45 materials including names, abbreviations, batch numbers suppliers and physical attributes.
| Name | Abbreviation | Batch number | Supplier |
|---|---|---|---|
| Microcrystalline Cellulose vivapur® type200 | MCC | 5620030813 | JRS Pharma GmbH&Co. KG |
| Microcrystalline Cellulose PH200NF | MCC | P0200J1511 | SSP, China-Germany joint venture |
| Microcrystalline Cellulose OricelTM PH-102 SCG | MCC | P0102F1506 | SSP, China-Germany joint venture |
| Microcrystalline Cellulose OricelTM PH302NF | MCC | P0102D1509 | SSP, China-Germany joint venture |
| Microcrystalline Cellulose OricelTM PH-112 | MCC | 5610237731 | SSP, China-Germany joint venture |
| MCC PH102 | MCC | NO.2565 | Asahi Kasei Chemicals Corporation |
| Microcrystalline Cellulose OricelTM PH302NF | MCC | P0302F1509 | SSP, China-Germany joint venture |
| Ethylcellulose N-7 Pharm | EC | 44156 | Ashland_Aqualon, Kentucky |
| Hydroxypropyl methylcellulose E5LV | HPMC | 3G190124L1 | The Dow Chemical Company |
| lactose Cellactose® 80 | Lac | L1533 | Molkerei Meggle Wasserburg GmBH & Co. KG |
| Croscarmellose sodium | CMC-Na | X5G248 | Nichirin chemical industries LTD |
| Hydroxy propyl methyl cellulose E3LV | HPMC | 2E150124L2 | The Dow Chemical Company |
| Calcium carboxymethylcellulose ECG505 | CMC-Ca | E4I086 | Nichirin chemical industries Ltd. |
| Radix Rehmanniae Recens extract | Sheng dihuang | SDH201609 | Beijing Tcmages Pharmaceutical Co., Ltd. |
| Herba Ecliptae extract | Mo Hanlian | MHL201609 | Beijing Tcmages Pharmaceutical Co., Ltd. |
| processed Radix Glycyrrhizae extract | Zhi gancao | ZGC201609 | Beijing Tcmages Pharmaceutical Co., Ltd. |
| Rhizoma Belamcandae extract | She gan | SG201609 | Beijing Tcmages Pharmaceutical Co., Ltd. |
| Radix Angelicae Sinensis extract | Dang gui | DG21609 | Beijing Tcmages Pharmaceutical Co., Ltd. |
| lactose Tablettose® 80 | Lac | L1543 | Molkerei Meggle Wasserburg GmBH & Co. KG |
| lactose Flowlac® 100 | Lac | L1517 | Molkerei Meggle Wasserburg GmBH & Co. KG |
| lactose Pharmatose® 110M | Lac | 1009CON | DFE Pharma |
| lactose anhydrous 21 AN | Lac | 1007NX8 | DFE Pharma |
| lactose Granulac® 200 | Lac | L1535 | Molkerei Meggle Wasserburg GmBH & Co. KG |
| lactose Spherolac® 100 | Lac | L1413 | Molkerei Meggle Wasserburg GmBH & Co. KG |
| lactose Granulac® 70 | Lac | L1533 | Molkerei Meggle Wasserburg GmBH & Co. KG |
| Croscarmellose sodium | CMC-Na | 10082Fo | DFE Pharma |
| Calcium phosphate dehydrate | / | 1286C350 | Avantor Performance Material Strading (Shanghai) Co.Ltd. |
| lactose anhydrous Duralac® H | Lac | LC042-5-805 | Molkerei Meggle Wasserburg GmBH & Co. KG |
| lactose Pharmatose® 200M | Lac | 10095MW | DFE Pharma |
| Pregelatinized starch | / | NO60820327 | Asahi Kasei Chemicals Corporation |
| Dextrin | / | 170307 | Beijing Biotopped Technology Co., Ltd. |
| Sodium bicarbonate | NaHCO3 | A038821 | Acros Organics Co., Ltd. |
| Calcium phosphate dibasic | CaHPO₄ | A0345625 | Acros Organics Co., Ltd. |
| Radix Scutellariae extract | Huang qin | HQ201609 | Beijing Tcmages Pharmaceutical Co., Ltd. |
| Radix Glycyrrhizae extract | Sheng gancao | SGC201609 | Beijing Tcmages Pharmaceutical Co., Ltd. |
| Rhizoma Ligustici extract | Chuan xiong | CX201609 | Beijing Tcmages Pharmaceutical Co., Ltd. |
| Carboxy methyl starch sodium | CMS-Na | C10072096 | Shanghai Macklin Biochemical Co.,Ltd. |
| Carboxy methyl starch sodium | CMS-Na | C10051641 | Shanghai Macklin Biochemical Co.,Ltd. |
| Croscarmellose sodium Vivasol® | CMC-Na | 3201061016 | JRS Pharma GmbH&Co. KG |
| Corn starch | / | MKBW7231V | Merck KGaA, Darmstadt |
| Radix Paeoniae Rubra extract | Chi shao | CS20180704 | Beijing Tcmages Pharmaceutical Co., Ltd. |
| Moutan Cortex extract | Mu danpi | MDP20180704 | Beijing Tcmages Pharmaceutical Co., Ltd. |
| Coptidis Rhizoma extract | Huang lian | HL20180704 | Beijing Tcmages Pharmaceutical Co., Ltd. |
| Radix Ploygoni Multiflori semi-extract | He shouwu | ZHSWB20190124 | Beijing Tcmages Pharmaceutical Co., Ltd. |
| Radix Ploygoni Multiflori blend | He shouwu | ZHSWH20190124 | Beijing Tcmages Pharmaceutical Co., Ltd. |
Cao, J.; Shen, H.; Zhao, S.; Ma, X.; Chen, L.; Dai, S.; Xu, B.; Qiao, Y. Sample Size Requirements of a Pharmaceutical Material Library: A Case in Predicting Direct Compression Tablet Tensile Strength by Latent Variable Modeling. Pharmaceutics 2024, 16, 242. https://doi.org/10.3390/pharmaceutics16020242
Read more on Direct Compression (DC) Excipients here:


