Multivariate Data Analysis and Central Composite Design-Oriented Optimization of Solid Carriers for Formulation of Curcumin-Loaded Solid SNEDDS: Dissolution and Bioavailability Assessment
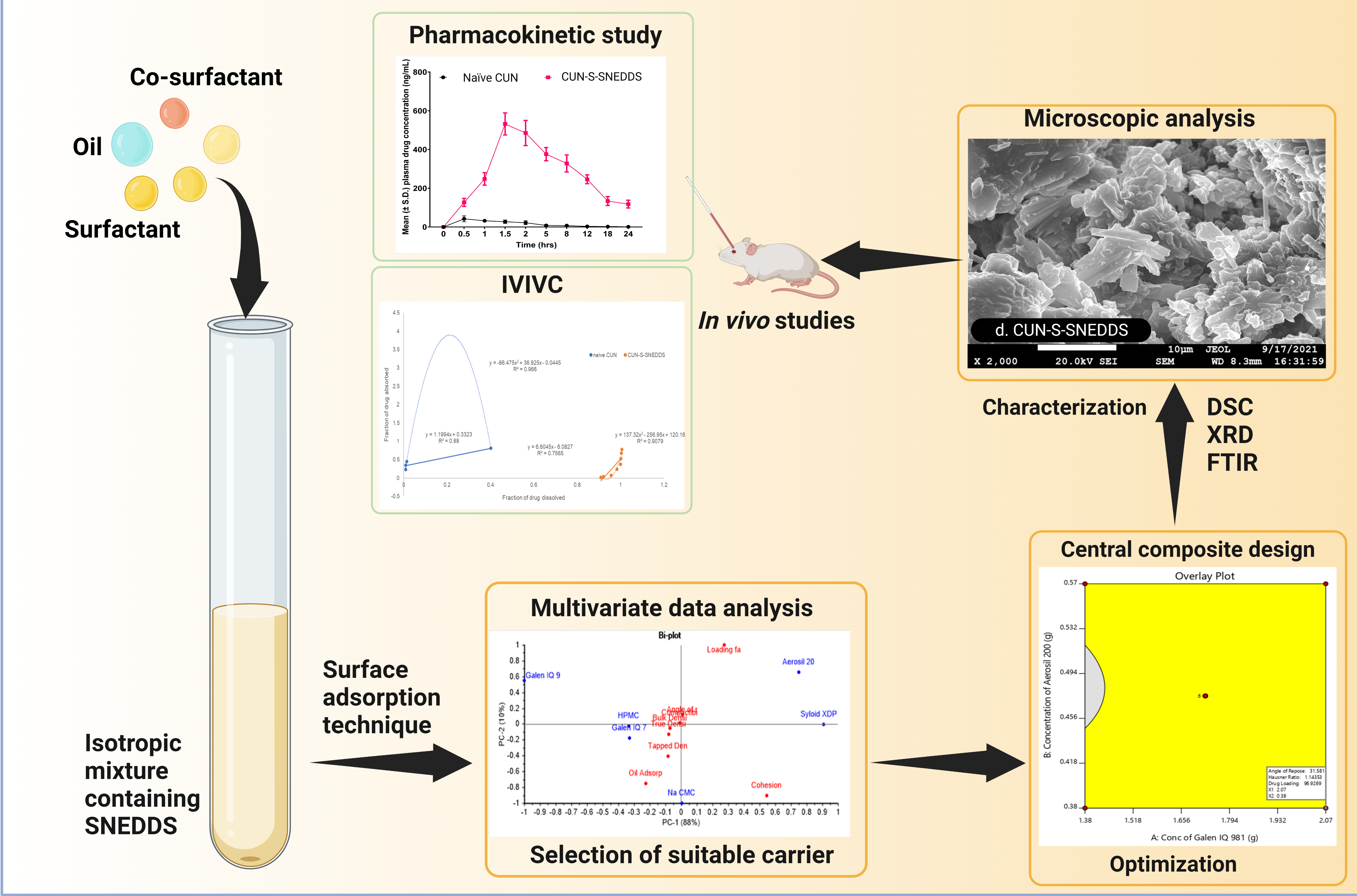
The study was initiated with two major purposes: investigating the role of isomalt (GIQ9) as a pharmaceutical carrier for solid self-nanoemulsifying drug delivery systems (S-SNEDDSs) and improving the oral bioavailability of lipophilic curcumin (CUN). GIQ9 has never been explored for solidification of liquid lipid-based nanoparticles such as a liquid isotropic mixture of a SNEDDS containing oil, surfactant and co-surfactant. The suitability of GIQ9 as a carrier was assessed by calculating the loading factor, flow and micromeritic properties. The S-SNEDDSs were prepared by surface adsorption technique. The formulation variables were optimized using central composite design (CCD). The optimized S-SNEDDS was evaluated for differential scanning calorimetry (DSC), Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), microscopy, dissolution and pharmacokinetic studies. The S-SNEDDS showed a particle size, zeta potential and PDI of 97 nm, −26.8 mV and 0.354, respectively. The results of DSC, XRD, FTIR and microscopic studies revealed that the isotropic mixture was adsorbed onto the solid carrier. The L-SNEDDS and S-SNEDDS showed no significant difference in drug release, indicating no change upon solidification. The optimized S-SNEDDS showed 5.1-fold and 61.7-fold enhancement in dissolution rate and oral bioavailability as compared to the naïve curcumin. The overall outcomes of the study indicated the suitability of GIQ9 as a solid carrier for SNEDDSs.
Download the full article as PDF here Multivariate Data Analysis and Central Composite Design-Oriented Optimization of Solid Carriers for Formulation of Curcumin-Loaded Solid SNEDDS_Dissolution and Bioavailability Assessment
or read it here
Materials
CUN was purchased from M/S Himedia, Mumbai, India. Propylene glycol (PG), Tween (T) 20 and 80; Polyethylene glycol (PEG) 200, 400 and 600: Span 20 and 80, Aerosil 200 (AER-200): hydroxy propyl methyl cellulose (HPMC); and sodium carboxy methyl cellulose (NaCMC) were purchased from Loba Chemie, Mumbai, India. Labrasol, Transcutol P (TRP) and Labrafil M1944 CS (LAB) were obtained as gift samples from M/s Gattefosse in Mumbai, India. Syloid XDP 3514 (SXDP) was obtained as a gift sample from Grace Materials Technologies, Discovery Science, Pune, India. Galen IQ 981 (GIQ9) and Galen IQ 721 (GIQ7) were obtained as gift samples from SFA Food and Pharma Ingredients, Thane, India. All other chemicals used were of analytical grade. In order the quantify the amount of drug, RP-HPLC was performed (SPDM20A; Shimadzu, Kyoto, Japan) at 420 nm, all experiments were carried out in triplicate and mean data were recorded.
Corrie, L.; Kaur, J.; Awasthi, A.; Vishwas, S.; Gulati, M.; Saini, S.; Kumar, B.; Pandey, N.K.; Gupta, G.; Dureja, H.; Chellapan, D.K.; Dua, K.; Tewari, D.; Singh, S.K. Multivariate Data Analysis and Central Composite Design-Oriented Optimization of Solid Carriers for Formulation of Curcumin-Loaded Solid SNEDDS: Dissolution and Bioavailability Assessment. Pharmaceutics 2022, 14, 2395. https://doi.org/10.3390/pharmaceutics14112395

