Development of a Solid Supersaturable Micelle of Revaprazan for Improved Dissolution and Oral Bioavailability Using Box-Behnken Design
Purpose: To enhance the oral bioavailability of revaprazan (RVP), a novel solid, supersaturable micelle (SSuM) was developed.
Methods: Surfactants and solid carriers were screened based on a solubility and a flowability test, respectively. Supersaturating agents, including Poloxamer 407 (P407), were screened. The SSuM was optimized using a Box-Behnken design with three independent variables, including Gelucire 44/14:Brij L4 (G44/BL4; X1) and the amounts of Florite PS-10 (FLO; X2) and Vivapur 105 (VP105; X3), and three response variables, ie, dissolution efficiency at 30 min (Y1), dissolution enhancing capacity (Y2), and Carr’s index (Y3). The solid state property was evaluated, and a dissolution test was conducted. RVP, Revanex®, solid micelle (P407-free from the composition of SSuM), and SSuM were orally administrated to rats (RVP 20 mg equivalent/kg) for in vivo pharmacokinetic study.
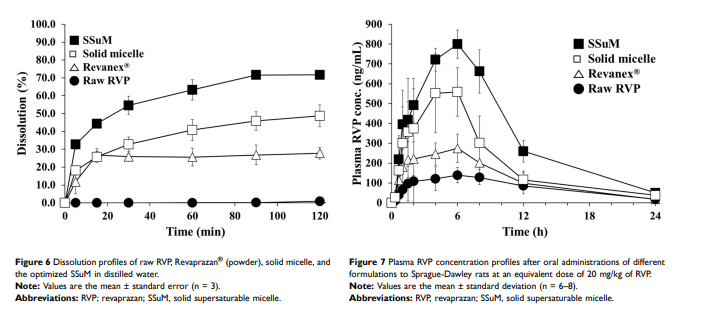
Results: G44 and BL4 showed great solubility, with a critical micelle concentration range of 119.2–333.0 μg/mL. P407 had an excellent supersaturating effect. FLO and VP105 were selected as solid carriers, with a critical solidifying ratio (g/mL) of 0.30 and 0.91, respectively. With optimized values of X1 (–0.41), X2 (0.31), and X3 (–0.78), RVP (200 mg)- containing SSuM consisting of G44 (253.8 mg), BL4 (106.2 mg), FLO (99.3 mg), VP105 (199.8 mg), and P407 (40 mg) was developed, resulting in Y1 (40.3%), Y2 (0.008), and Y3 (12.3%). RVP existed in an amorphous state in the optimized SSuM, and the SSuM formed a nanosized dispersion in the aqueous phase, with approximately 71.7% dissolution at 2 h. The optimized SSuM improved the relative bioavailability of RVP in rats by approximately 478%, 276%, and 161% compared to raw RVP, Revanex®, and solid micelle, respectively.
Conclusion: The optimized SSuM has great potential for the development of solidified
formulations of poorly water-soluble drugs with improved oral absorption.
Download the full research paper here: Development-of-a-solid-supersaturable-micelle-of-revaprazan
Materials
RVP powder (purity >99%) and Revanex® tablets containing 200 mg RVP were supplied by Yuhan (Seoul, Korea).
Aerosil® 200 was supplied by Evonik Degussa GmbH (Frankfurt am Main, Germany). Avicel PH 101, Brij L4 (BL4), Tween® 20, and Tween® 80 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Florite PS-10 (FLO; calcium silicate) was purchased from Tomita Pharmaceutical Co., Ltd. (Tokushima, Japan). Gelucire 44/14 (G44), Labrafil® M 1944 CS, Labrasol®, and Lauroglycol® 90 were gifted by Gattefossé (Saint Priest, France). Kolliphor® EL, Kolliphor® RH40, Kolliphor® RH60, Kollidon K30, Kollidon K90, Poloxamer (P)188, and P407 were obtained from BASF (Ludwigshafen, Germany). Low-substituted hydroxypropyl cellulose B1 (L-HPC) was supplied by Shin-Etsu Chemical Co., Ltd. (Tokyo, Japan). Neusilin® US2 (magnesium aluminometasilicate) was supplied by Fuji Chemical Industry Company (Toyama, Japan). Sylysia® 350 (porous silica) was supplied by Fuji Silysia Chemical Co., Ltd. (Aichi, Japan). Vivapur (VP)12 and VP105 were supplied by JRS Pharma (Rosenberg, Germany)
Keywords: revaprazan, supersaturation, solid micelle, Box-Behnken design, dissolution,
oral bioavailability
Yoon Tae Goo, Cheol-Ki Sa , Ji Yeh Choi, Min Song Kim, Chang Hyun Kim, Hyeon Kyun Kim, Young Wook Choi
International Journal of Nanomedicine, 2021:16 1245–1259

