Statistically developed stable Camptothecin-loaded Soluplus/TPGS mixed micelles for improved ovarian cancer treatment
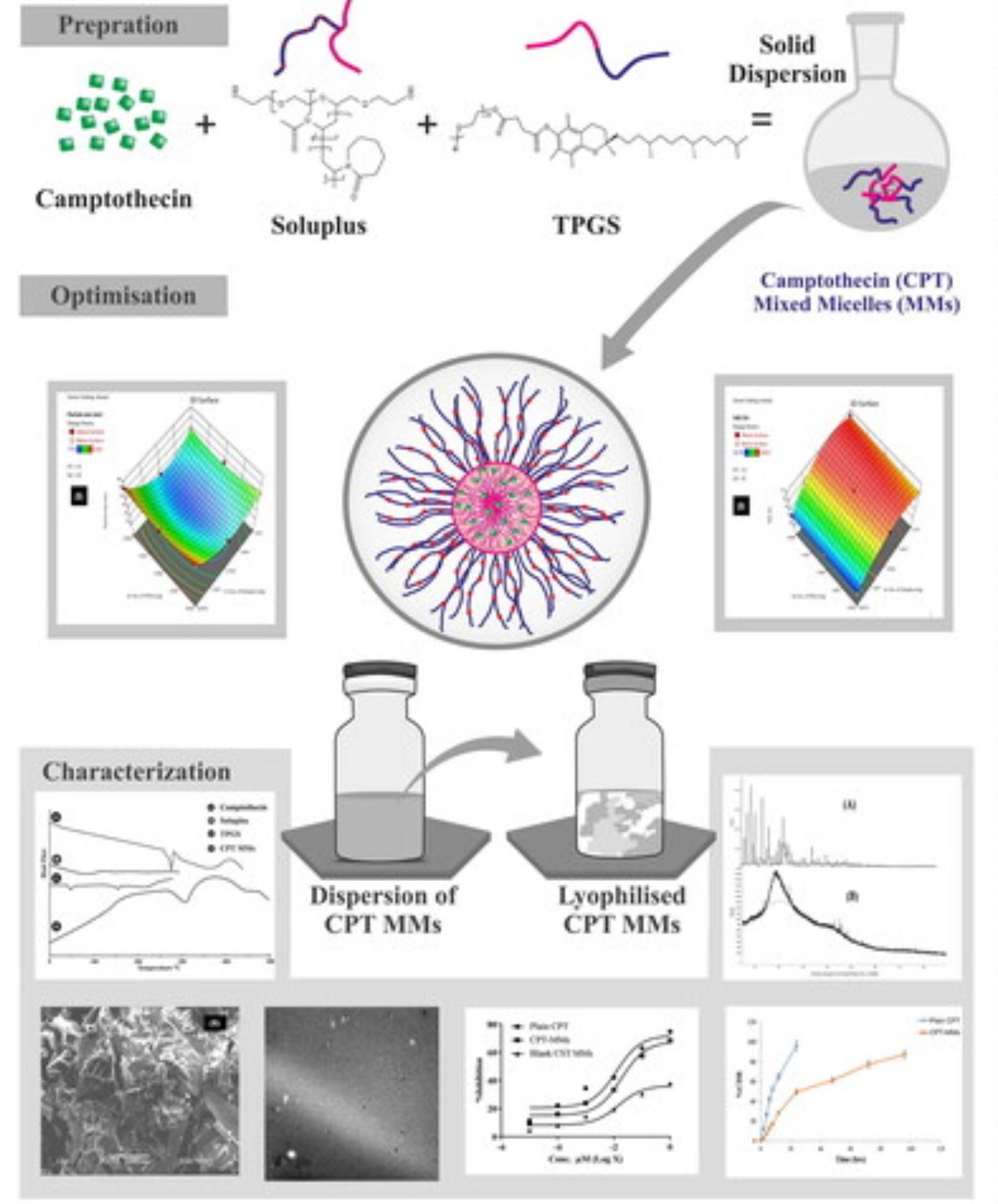
In this research, Camptothecin-loaded mixed micelles (CPT-MMs) were formulated by combining Soluplus®, a novel amphiphilic excipient, with D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS). The use of appropriate excipients is crucial in stabilizing micellar structures and improving drug entrapment efficiency. TPGS, with its ability to self-assemble into micelles, was an ideal candidate, and incorporating Soluplus further enhanced micelle stability and drug-loading capacity. A 32 full factorial design was employed to investigate the influence of key factors on particle size and %entrapment efficiency (%EE).
The statistical analysis using Design Expert® VR software provided meaningful conclusions on the significance of each factor and their interactions, facilitating the successful optimization of CPT-MMs dispersion with improved drug delivery capabilities. The mean particle size, zeta potential, %drug loading capacity (%DLC), and (%EE) of CPT-MMs were 77.8 ± 2.67 nm, −23.4 ± 0.36 mV, 45.6 ± 1.78%, and 68.61 ± 1.8% respectively. CPT-MMs demonstrated sustained-release characteristics, preventing premature drug release and enhancing drug accumulation in tumor tissues, potentially optimizing CPT delivery for cancer treatment. Importantly, CPT-MMs dispersion exhibited lower hemolysis compared to plain CPT, indicating excellent biocompatibility and safety for intravenous administration.
The sustained release of CPT from CPT-MMs dispersion may lead to reduced cytotoxicity and improved therapeutic efficacy. Additionally, the lyophilized formulation showed superior stability compared to the liquid micelles. In conclusion, the development of CPT-MMs presents a promising strategy for enhancing drug delivery and optimizing therapeutic outcomes in cancer treatment. The sustained-release feature, along with enhanced biocompatibility and stability, holds great potential for the clinical translation of these MMs as efficient drug delivery systems for ovarian cancer treatment.
Read more here
Shruti Kargane, Rutuja Chougale, Nitin Mali, Kiran Patil, Shalaka Patki & John Disouza, Statistically developed stable Camptothecin-loaded Soluplus/TPGS mixed micelles for improved ovarian cancer treatment, Received 08 Aug 2023, Accepted 08 Jan 2024, Published online: 12 Feb 2024, https://doi.org/10.1080/01932691.2024.2304634
Read more on TPGS here and watch also the interesting video on Vitamin E TPGS:

