Surfactants for stabilization of dermal emulsions and their skin compatibility under UVA irradiation: Diacyl phospholipids and polysorbate 80 result in high viability rates of primary human skin cells
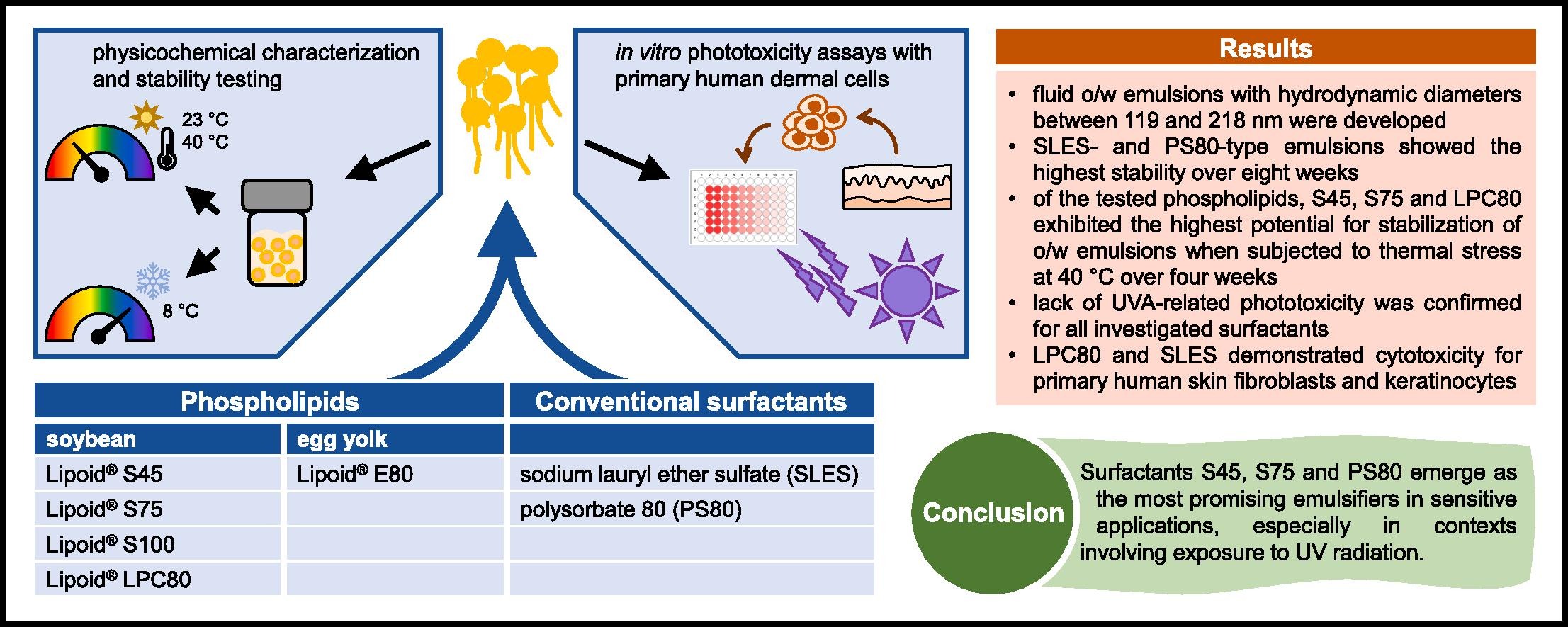
Phospholipids are versatile formulation compounds with high biocompatibility. However, no data on their effect on skin in combination with UVA radiation exist. Thus, it was the aim of this work to (i) develop o/w nanoemulsions (NEs) differing in surfactant type and to investigate their physicochemical stability at different storage temperatures, (ii) establish a standardized protocol for in vitro phototoxicity testing using primary human skin cells and (iii) investigate the phototoxicity of amphoteric phospholipids (S45, S75, E80, S100, LPC80), sodium lauryl ether sulfate (SLES) and polysorbate 80 (PS80). Satisfying systems were developed with all surfactants except S100 due to low zeta potential (-21.4 mV ± 4.69).
SLES and PS80-type NEs showed the highest stability after eight weeks; temperature-dependent variations in storage stability were most noticeable for phospholipid surfactants. For phospholipid-based NEs, higher phosphatidylcholine content led to unstable formulations. Phototoxicity assays with primary skin fibroblasts confirmed the lack of UVA-related phototoxicity but revealed cytotoxic effects of LPC80 and SLES, resulting in cell viability as low as 2.7 % ± 0.78 and 1.9 % ± 1.57 compared to the control. Our findings suggest that surfactants S45, S75 and PS80 are the most promising candidates for skin-friendly emulsifiers in sensitive applications involving exposure to UV light.
Download the full article as PDF here: Surfactants for stabilization of dermal emulsions and their skin compatibility under UVA irradiation
or read it here
Materials
Phospholipid-based emulsifiers Lipoid® S45, S75, S100, E80 and P-LPC80 (Fig. 1B) were kindly provided by Lipoid GmbH (Ludwigshafen, GER). Sodium lauryl ether sulfate (SLES) was obtained from Caesar & Loretz GmbH (Hilden, GER). Medium-chain triglycerides (MCT) were purchased from Herba Chemosan (Vienna, AUT). Polysorbate 80 (PS80, Tween®80), neutral red (NR) and chlorpromazine hydrochloride (CPZ) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s Modified Eagle’s Medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco by Life Technologies Limited (Paisley, UK). Penicillin-Streptomycin was obtained from Biowest SAS (Nuaillé, FR). Keratinocyte Growth Medium 2 (KGM-2) was purchased from Biomedica Medizinprodukte GmbH (Vienna, Austria).
Katja Steiner, Jakob Josef Schmolz, Felisa Hoang, Hanna Wolf, Saskia Seiser, Adelheid Elbe-Bürger, Victoria Klang,
Surfactants for stabilization of dermal emulsions and their skin compatibility under UVA irradiation: Diacyl phospholipids and polysorbate 80 result in high viability rates of primary human skin cells, International Journal of Pharmaceutics, Volume 653, 2024, 123903, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.123903.
See also the following articles on Surfactants here:
- Sustained release polymer and surfactant based solid dispersion of andrographolide exhibited improved solubility, dissolution, pharmacokinetics, and pharmacological activity
- Design of Redispersible High-Drug-Load Amorphous Formulations: Impact of Ionic vs Nonionic Surfactants on Processing and Performance
- Safety of Surfactant Excipients in Oral Drug Formulations