Development, Physicochemical Characteristics and Pharmacokinetics of a New Sustained-Release Bilayer Tablet Formulation of Tramadol with an Immediate-Release Component for Twice-Daily Administration
Abstract
Background and Objective
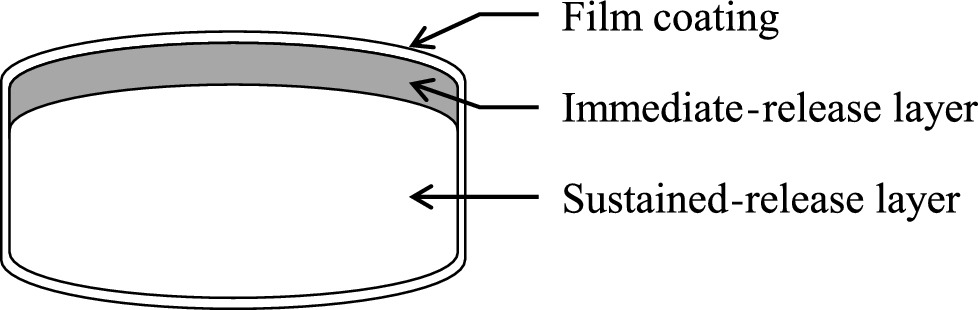
There are some potential concerns about the currently marketed solid oral dosage forms of tramadol, including decreased adherence to immediate-release (IR) formulations due to the high number of doses taken each day and the slow rise in the blood tramadol concentration after administration of sustained-release (SR) formulations, which may not achieve a rapid analgesic effect. To overcome these potential concerns, a twice-daily double-layered tablet formulation of tramadol comprising IR and SR layers was developed. This article reports studies that assessed its physicochemical and pharmacokinetic properties.
Methods
Dissolution tests of five bilayer tablet formulations (designated tablets A–E) and pharmacokinetic studies of tablets A and B were conducted to investigate the appropriate ratio of the IR/SR layers in the double-layered tablet. Additionally, pharmacokinetic studies of three finished dosage formulations (tablets C–E) were performed in healthy adult males to investigate the effect of food intake on drug absorption.
Results
Adjusting the excipients and tramadol content in the IR and SR layers of tablets A–E altered their dissolution profiles in a manner that could be predicted based on their compositions. The IR layer was released within 15 min, and the SR layer was slowly released over 10 h. In the pharmacokinetic study, the time to maximum plasma concentration (tmax) of tramadol after administration of tablets A (IR:SR: 20:80 mg) and B (40:60 mg) was shorter than that of a commercially available SR tablet, and the half-life (t1/2) was longer than that of a commercially available IR tablet. For tablets C–E, administration after food did not affect the area under the concentration-time curve (AUC) or maximum drug concentration (Cmax) of tramadol, but the tmax was prolonged by about 1 h compared with administration in fasting conditions. The mean ± standard deviation tmax and t1/2 for tablet D (IR:SR: 35:65 mg) in the fasting condition was 1.09 ± 0.56 h and 7.82 ± 0.85 h, respectively. The respective values in the fed condition were 2.47 ± 1.06 h and 7.12 ± 0.85 h, respectively.
Conclusions
To address the potential concerns regarding existing formulations of tramadol, a twice-daily, extended-release bilayer formulation of tramadol consisting of an IR and SR layer was developed. Pharmacokinetic studies confirmed that the plasma tramadol concentration increased quickly after administration and was maintained over a long period of time.
2.1 Materials
Tramadol was manufactured by Cadila Healthcare Ltd. (India) and milled and powdered for use. It was combined with the following additives: lactose hydrate (LH; DFE Pharma, The Netherlands; disintegration aid), microcrystalline cellulose (MCC; Asahi Kasei, Japan; binder) and erythritol (Nikken Chemical, Japan; dissolution aid) as excipients; croscarmellose sodium (CCMC-Na; DuPont, USA) and partly pregelatinized starch (PCS; Asahi Kasei) as disintegrators; pullulan (Hayashibara, Japan), hydroxypropyl cellulose-SL (HPC-SL; Nippon Soda, Japan), carboxyvinyl polymer (CVP; Lubrizol, USA), hydroxypropyl cellulose-H (HPC-H; Nippon Soda) and carmellose sodium (CMC-Na; Daicel Miraizu, Japan) as binders; magnesium stearate (MgSt; Taihei Chemical Industrial, Japan) as a lubricant; hypromellose (HM; Shin-Etsu Chemical, Japan) and macrogol 6000 (PEG6000; macrogol 6000P, NOF Corp., Japan) as a plasticizer; and yellow and red ferric oxide (Kishi Kasei, Japan) as coloring agents. Tramadol, MCC, CCMC-Na, HPC-SL, HPC-H, pullulan, CMC-Na, MgSt, HM and PEG6000 all conformed to the Japanese Pharmacopoeia [15].
Download the full article as PDF here: Development, Physicochemical Characteristics and Pharmacokinetics of a New Sustained-Release Bilayer Tablet Formulation of Tramadol with an Immediate-Release Component for Twice-Daily Administration
or read it here
Ishitsubo, N., Oguro, S., Shimahashi, H. et al. Development, Physicochemical Characteristics and Pharmacokinetics of a New Sustained-Release Bilayer Tablet Formulation of Tramadol with an Immediate-Release Component for Twice-Daily Administration. Eur J Drug Metab Pharmacokinet (2023).
https://doi.org/10.1007/s13318-023-00865-1

